Question
Question: Which of the following is the strongest base? A) 
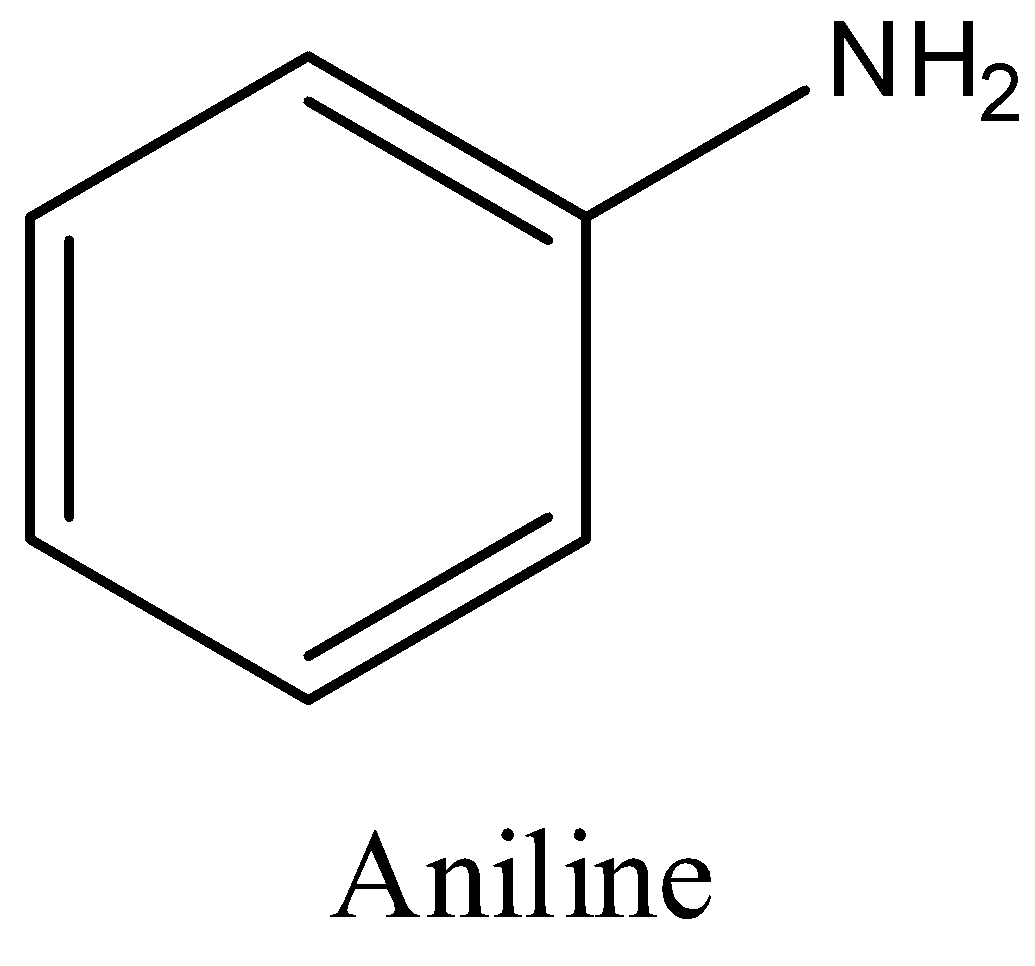
B)
C)
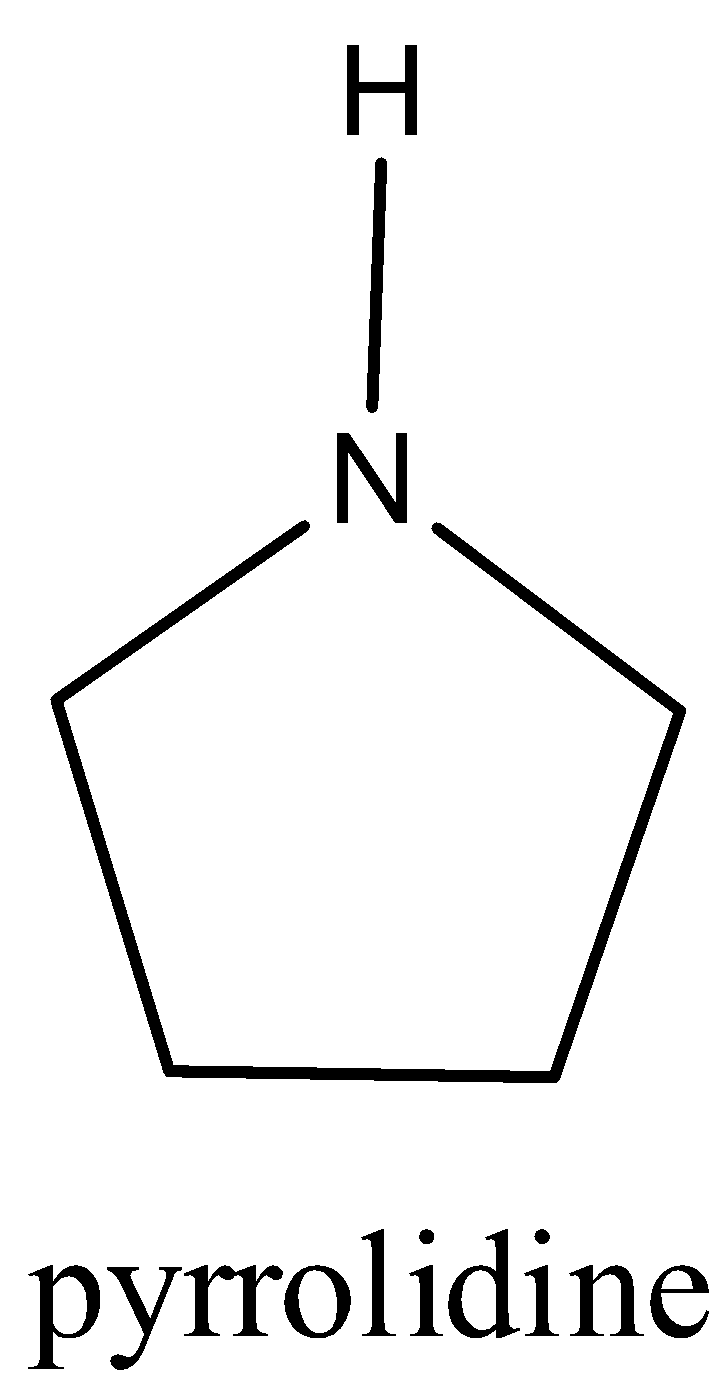
D)
Solution
We know in science, there are three definitions in like manner utilization of the word base, known as Arrhenius bases, Bronsted bases and Lewis bases. All definitions concur that bases are substances which respond with acids as initially proposed by G.- F. Rouelle during the eighteenth century. Svante Arrhenius proposed in 1884 that a base is a substance which separates in fluid answers for structure hydroxide particlesOH−. These particles can respond with hydrogen particles from the separation of acids to shape water in a corrosive base response.
Complete answer:
We have to know that Pyrrolidine is a completely diminished Pyrrole and piperidine is a completely diminished pyridine. Presently pyrrolidine has a five membered ring while piperidine has a six membered ring. ... Consequently the form corrosive of pyrrolidine is somewhat more balanced out over piperidine and henceforth pyrrolidine has a marginally higher basicity over piperidine. Pyrrolidine is more essential than aniline. Overall alicyclic amines are more essential than fragrant amines. In aniline, a solitary pair of electrons are associated with reverberation with the ring and consequently not promptly accessible for resonance.
Hence option C is correct.
Note:
We have to know that bases and acids are viewed as synthetic alternate extremes on the grounds that the impact of a corrosive is to build the hydronium focus in water, while bases lessen this fixation. A response between watery arrangements of a corrosive and a base is called balance, creating an answer of water and a salt in which the salt isolates into its segment particles. In the event that the fluid arrangement is immersed with a given salt solute, any extra such salt is encouraged out of the arrangement. A base was consequently a metal hydroxide like NaOH or Ca(OH)2 . Such fluid hydroxide arrangements were additionally portrayed by certain trademark properties. They are dangerous to the touch, can taste bitter and change the shade of pH markers (e.g., become red litmus paper blue).
