Question
Question: Which of the following is the strongest base? a)  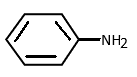
b) 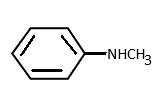
c) 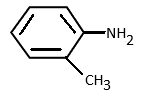
d) 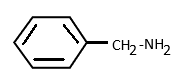
Solution
The basicity depends upon the tendency of a base to donate its lone pair of electrons to accept a proton. This nature can be increased if some electron-containing groups are attached alongside.
Complete step by step solution:
The Amino group acts as a strong base as the nitrogen attached has one lone pair of electrons. The electronegativity of nitrogen is also not so high, thus it can donate its lone pair easily.
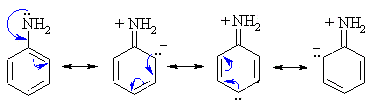
As we know, the benzene ring is an electron rich species with three alternate double bonds. When Amino group is attached to a benzene ring, the carbon is sp2 hybridised. Thus as sp2 carbon is more electronegative than sp3 nitrogen, the lone pair of nitrogen are delocalised in a ring having few resonating structures. Hence aniline is not very basic.
N-methyl benzamide is a secondary amine. Here also the lone pair of electrons of nitrogen is delocalised in the ring and thus less available for donation. Hence option (b) it is also not a strong base.
O-toluidine has a methyl group on the ortho position of the amino group. Thus methyl will show +I effect. But the lone pair on nitrogen experiences repulsion from the adjacent hydrogen attached to the methyl group, also known as steric repulsion and will have very less basicity.
In option (d), the carbon attached to nitrogen is sp3 hybridised. Hence as sp3 carbon has very low electronegativity, the lone pair on nitrogen are localised and not attracted to carbon or benzene rings. Thus benzyl amine has the strongest basic nature among all.
∴ Option (d) is correct.
Note:
Basicity of a compound depends on the tendency to donate lone pairs of electrons. If the Kb of a base is very high and pKb value is low ten the base is considered as a strong base.
