Question
Question: Which of the following is the strongest base? (A) 
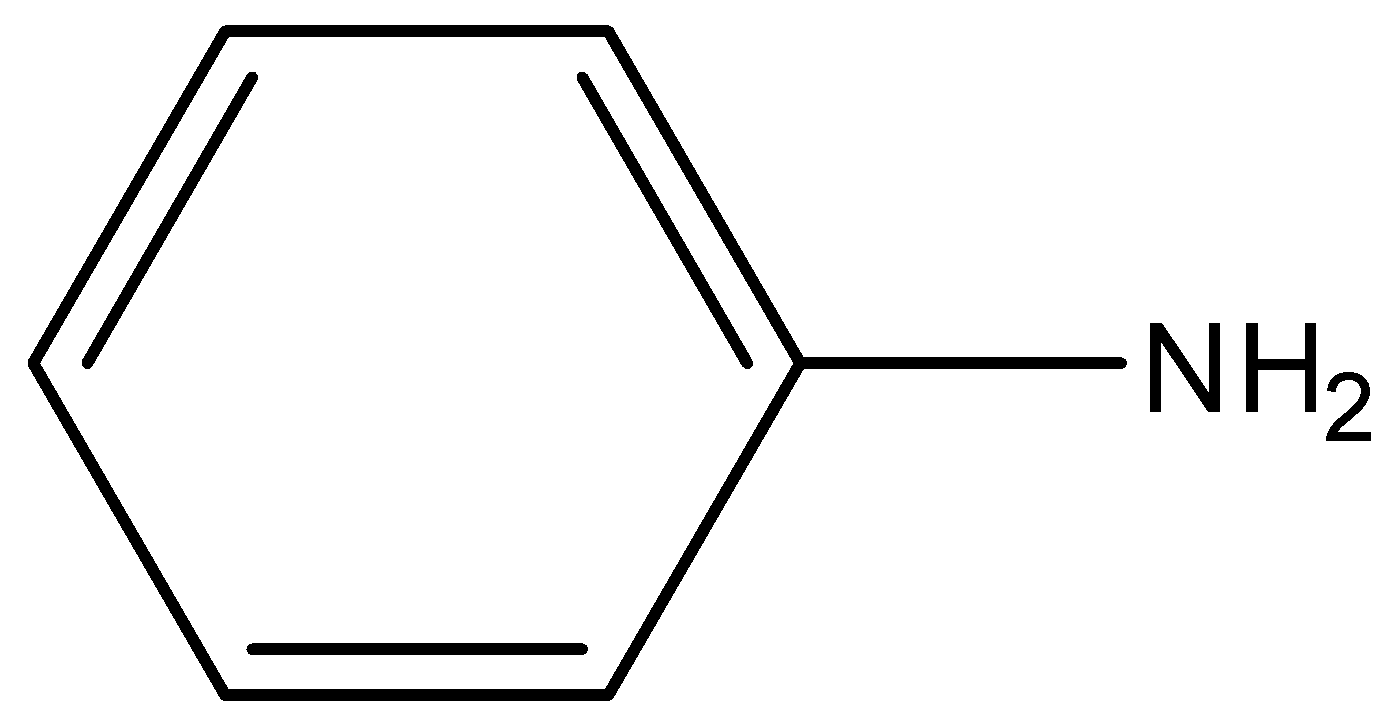
(B)
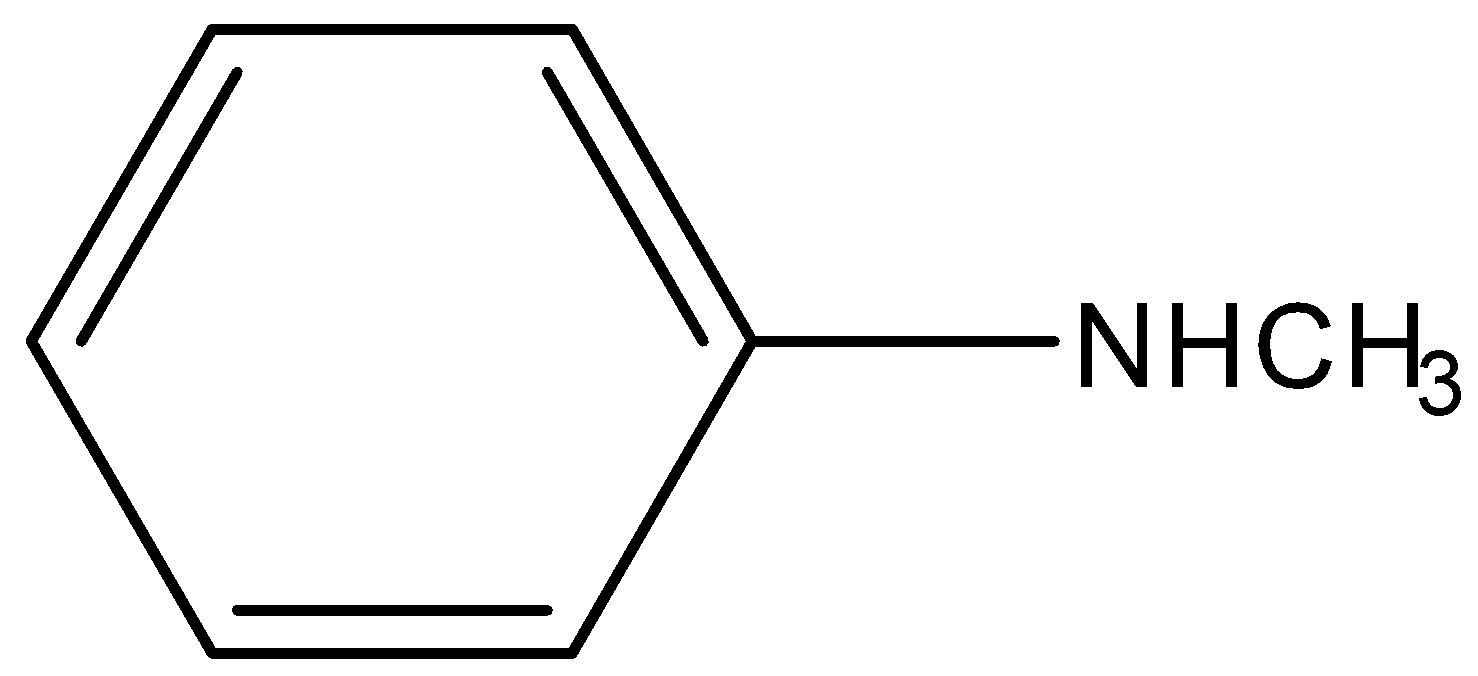
(C)
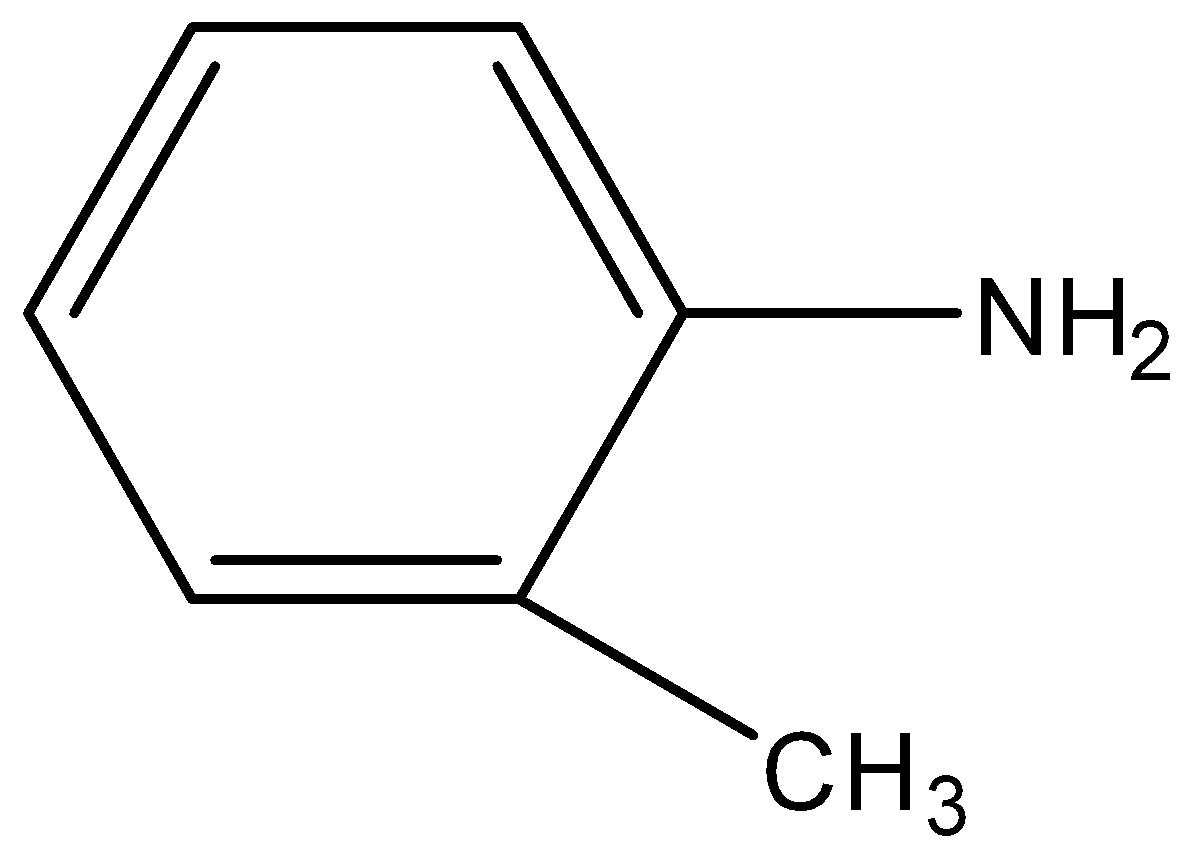
(D)
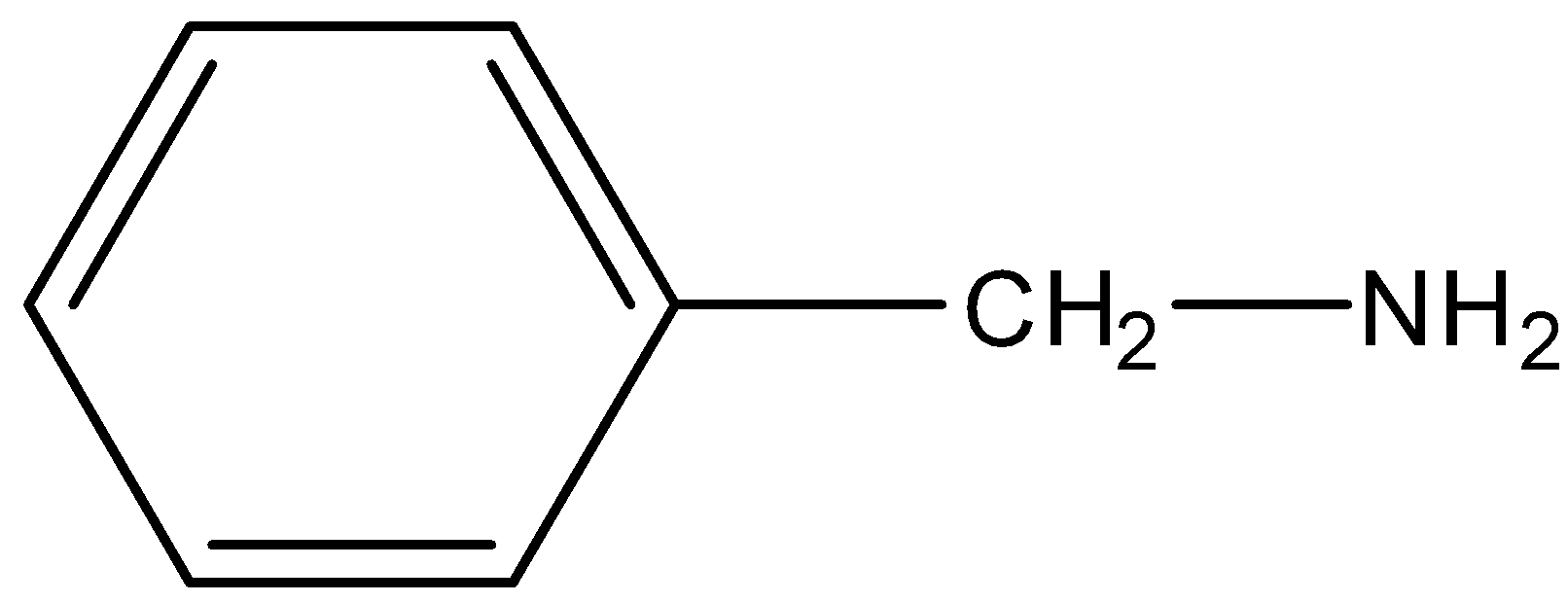
Solution
Arrhenius gave the concept of bases and told us that bases are those species which dissociates in an aqueous solution to give hydroxide ion (OH−). The Bronsted-Lowry told us that bases are those that can accept hydrogen cations i.e (H+). Strong bases are those bases in which lone pairs of electrons are available for donation to acids.
Complete step by step answer:
-Strong bases are those that can remove a proton even from a very weak acid, say for example water in acid-base reactions. Bases turn red litmus paper to blue. Common example of a strong base is sodium hydroxide also known as caustic soda.
-In case of aniline, the lone pair is present on the nitrogen atom. It is in resonance with the aromatic ring that is benzene. It is not available for donation. So, it’s unavailable for donation.
-In case of N-methyl aniline, the electrons present on nitrogen atoms are also not available for donation because they are in conjugation with an aromatic ring. So it is not a strong base. Hence it is also unavailable for donation.
-In the case of O-toluidine, nitrogen is directly attached to the aromatic ring but not to any other compound so the lone pair on nitrogen is in resonance with the benzene ring and not available for donation. It is not a strong base so it can’t donate the electrons.
-In the case of Benzyl amine, the nitrogen is attached to the methyl group in this compound. So nitrogen can donate its lone pair to the methyl group. So it will have free electrons for donation. Thus it is a strong base.
-Hence Benzyl amine is the strongest base among given compounds.
So, the correct answer is “Option D”.
Note:
Strong bases react violently with acids and they have free electrons for donation. Strong bases have low solubility. Strong bases completely hydrolyse in water. They donate their electrons and dissociate completely.
