Question
Question: Which of the following is the smallest ring that can accommodate a triple bond? (A) Cyclohexyne ...
Which of the following is the smallest ring that can accommodate a triple bond?
(A) Cyclohexyne
(B) Cycloheptyne
(C) Cyclooctyne
(D) Bicyclononyne
Solution
As we know that triple bond can be carried by a compound if it has a linear structure in case of open chain structures or minimal angle strain in case of cycloalkynes. Hence we must attempt this question by checking the angle strain in the compound and whether it is suitable for triple bond or not.
Complete step-by-step answer: First we must know a bit about alkynes and cycloalkynes:-
-Alkynes are the unsaturated hydrocarbons containing at least one carbon-carbon (R−C≡C−R′ ) triple bond. Its shape is linear and forms 180∘ with the adjacent R-groups attached to it.
-In chemistry, a cycloalkyne is the cyclic analog of a straight chain containing a triple bond (alkyne). It consists of a closed ring of carbon atoms which may have one or more triple bonds.
-Since the nature of R−C≡C−R′ alkyne unit is linear, cycloalkynes can be highly strained and may only exist in cases where the number of carbon atoms in the ring is high enough to provide the flexibility which would be necessary to accommodate this geometry and bond angle.
(A) Cyclohexyne (6-member ring): This structure cannot be isolated (only exist as transient intermediate) as it has non negligible angle strain which may not be able to accommodate triple bond and come to existence.
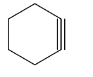
(B) Cycloheptyne (7-member ring): Similarly in this case, it cannot be isolated (only exist as transient intermediate) as it has non negligible angle strain which may not be able to accommodate triple bond and come to existence.
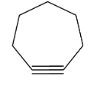
(C) Cyclooctyne (8-member ring): Here the angle strain is quite negligible and hence it may accommodate triple bond; cyclooctyne is isolable and very reactive in nature.
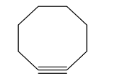
(D) Bicyclononyne (9-member ring): Similarly here also the angle strain is quite negligible and hence it may accommodate triple bond; cyclononyne is easily isolable and very reactive in nature. Since this ring is greater than cyclooctyne, hence more flexible.
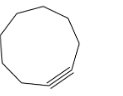
-So from the above data we may conclude that cyclooctyne is the smallest ring to accommodate triple bonds.
Therefore, the correct option is: (C) Cyclooctyne.
Note: -We may often get confused that benzyne is a 6 member ring and it may exist as we use it in various reactions but it is a wrong assumption. Benzyne is a reaction intermediate and it cannot be isolated during the reactions.
-there is no evidence regarding 3, 4 or 5-member rings with triple bonds as these are highly strained systems.
