Question
Question: Which of the following is the most substrate most reactive towards methoxide ions? A.\(C{H_3} - I\...
Which of the following is the most substrate most reactive towards methoxide ions?
A.CH3−I
B.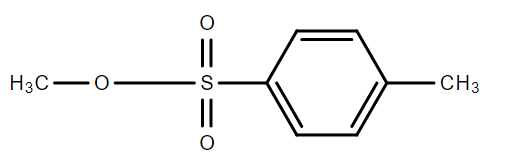
C.CH3−O−SO2−CF3
D.CH3−F
Solution
To answer this question, you should recall the concept of nucleophilic substitution reactions. The substitution reaction is the type of reaction where a functional group of one chemical compound is substituted by another group or it is a reaction which involves the replacement of one atom or a molecule of a compound with another atom or molecule.
Complete step by step answer:
Methoxide is a strong base hence, will react by the process of SN2 or E2. Since both of these pathways are concerted, each has only one step. Therefore, the rate-limiting transition state given by the rate law is the only transition state of the reaction.
The stereochemistry of each mechanism will be tested by making the α -carbon a stereocenter. These pathways share a great number of similarities. Both require a good leaving group. SN2 reactions require a good nucleophile, while E2 reactions require a good base.
In most cases, however, a good nucleophile is also a good base. Thus SN2 and E2 often compete in the same reaction conditions. The winner is determined by the degree of α and β branching and the strength of the nucleophile/base. Increased α and β branching and strong basicity favour E2 elimination. Increased nucleophilicity favours the SN2 reaction.
Using the above information, we can see that the best option resulting in stable products is option C.
The reaction can be represented as:
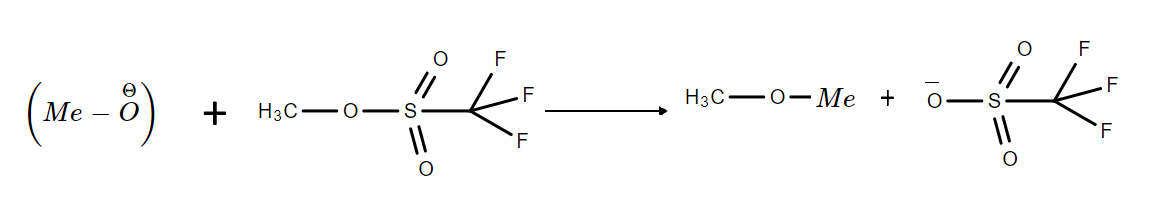
Hence, the correct option is C.
Note:
Make sure you remember the difference between SN1 and SN2 reaction mechanisms. SN1 involves the formation of a carbocation intermediate which is generally in case of tertiary or secondary alkyl halides as their intermediates are stabilised by hyperconjugation with secondary or tertiary alcohols under strongly acidic or strongly basic conditions. The SN1 mechanism also is known as a dissociative mechanism. In SN2 the reaction mechanism, the nucleophile approaches the given substrate at an angle of180o to the carbon-leaving group bond. Now, the leaving group is pushed out of the transition state on the opposite side of the carbon-nucleophile bond, forming the required product. It is important to note that the product is formed with an inversion of the tetrahedral geometry at the atom in the centre.
