Question
Question: Which of the following is the most stable cycloalkane? (A) Cyclopropane (B) Cyclobutane (C) Cy...
Which of the following is the most stable cycloalkane?
(A) Cyclopropane
(B) Cyclobutane
(C) Cyclopentane
(D) Cyclohexane
Solution
In the above question to know the stability of the cycloalkane we have to know the angle strain in the different cycloalkanes. The understanding of the angle strain would come from the “Baeyer strain Theory”. This theory tells that any deviation of bond angle from the normal tetrahedral value would impose a condition of internal strain in the ring.
Complete step-by-step answer: To understand strain we can take one example of Cyclopropane.
In cyclopropane, all the three carbons occupy the corners of the equilateral triangle. And thus cyclopropane has c−c−cbond angle of 60∘.
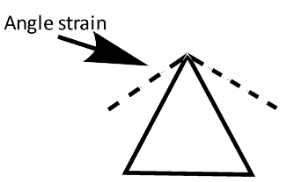
But, normal tetrahedral bond angle between c−c−c should be 109∘28′. This implies that normal tetrahedral angle of 109∘28′ between two bonds is compressed to 60∘and each of the two bonds is pulled in by:
Angle strain = 21(109∘28′−60∘)=24∘44′.
The value 22∘44′represents the angle strain in the cyclopropane. Similarly, the angle strain in the other cycloalkane can be calculated the same way. Hence, I gave the table for angle strain for our options in question.
\begin{array}{*{20}{c}}
{Compound}&{Bond - angle}&{Angle - strain} \\\
{Cyclopropane}&{60^\circ }&{22^\circ 44'} \\\
{Cyclobutane}&{90^\circ }&{9^\circ 44'} \\\
{Cyclopentane}&{108^\circ }&{0^\circ 44'} \\\
{Cyclohexane}&{120^\circ }&{ - 5^\circ 16'}
\end{array}
The angle strain is the minimum in case of cyclopentane. Hence, according to the Baeyer strain theory cyclopentane has lower strain in its ring therefore, it is a more stable molecule.
Hence,option (C) is correct.
Note: In this type of question students are aware about the angle between two carbon atoms in the ring. One more thing is that after a six membered cyclic ring the strain increases with increase in the number of carbons. So, the stability is also decreasing according to the Baeyer strain theory.
