Question
Question: Which of the following is the most correct electron displacement for a nucleophilic reaction to take...
Which of the following is the most correct electron displacement for a nucleophilic reaction to take place?
A)
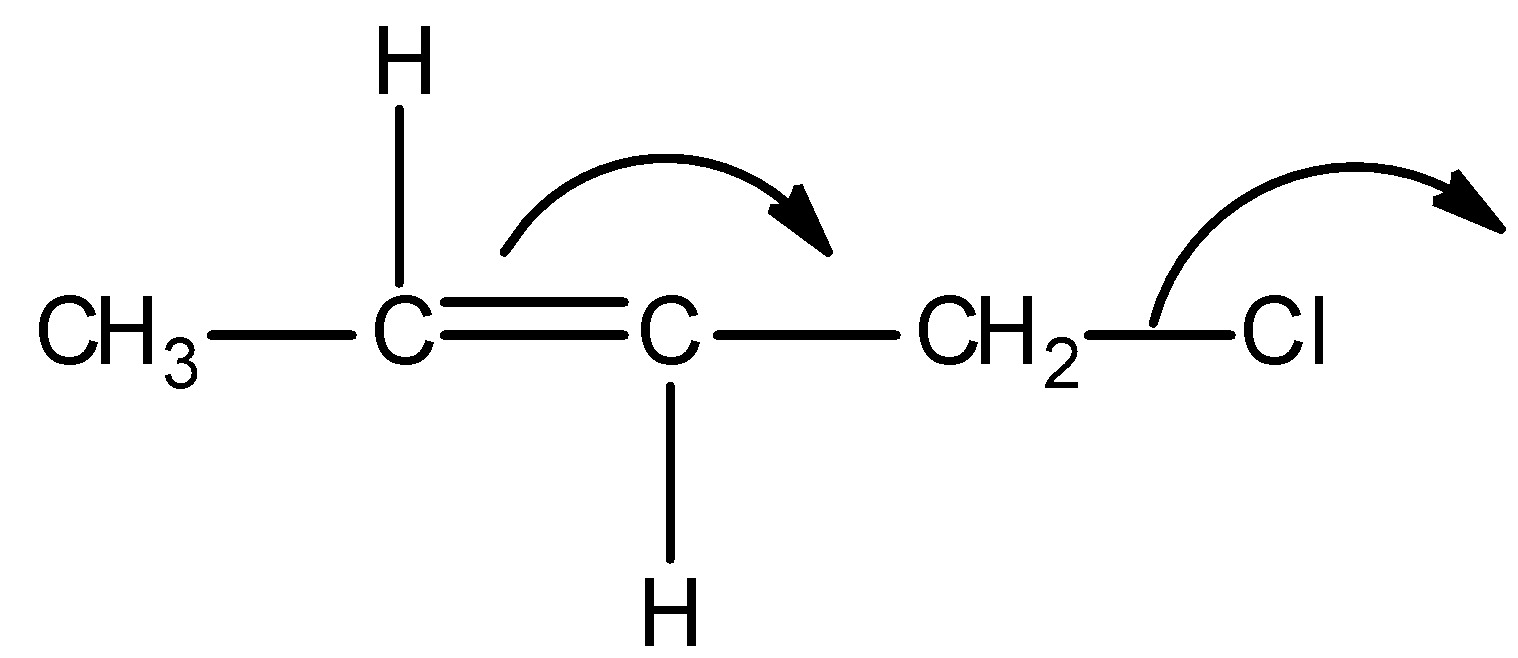
B)
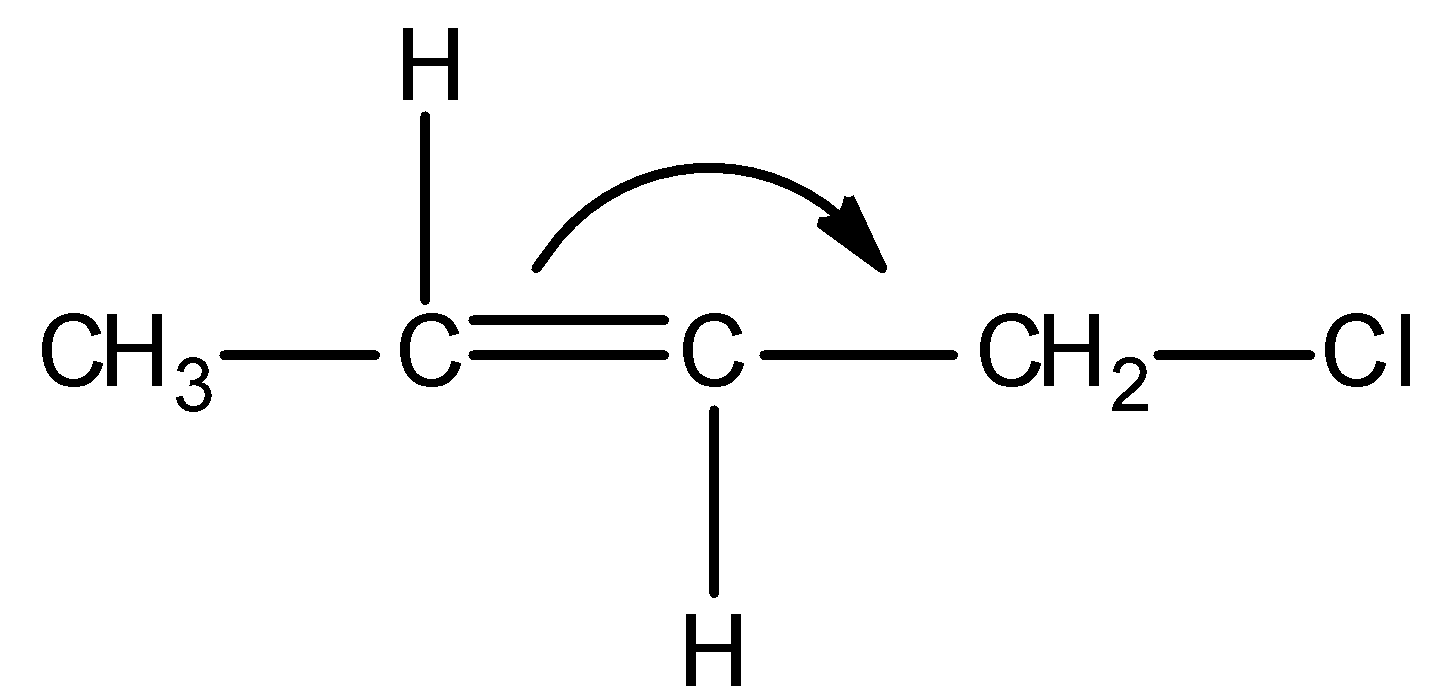
C)
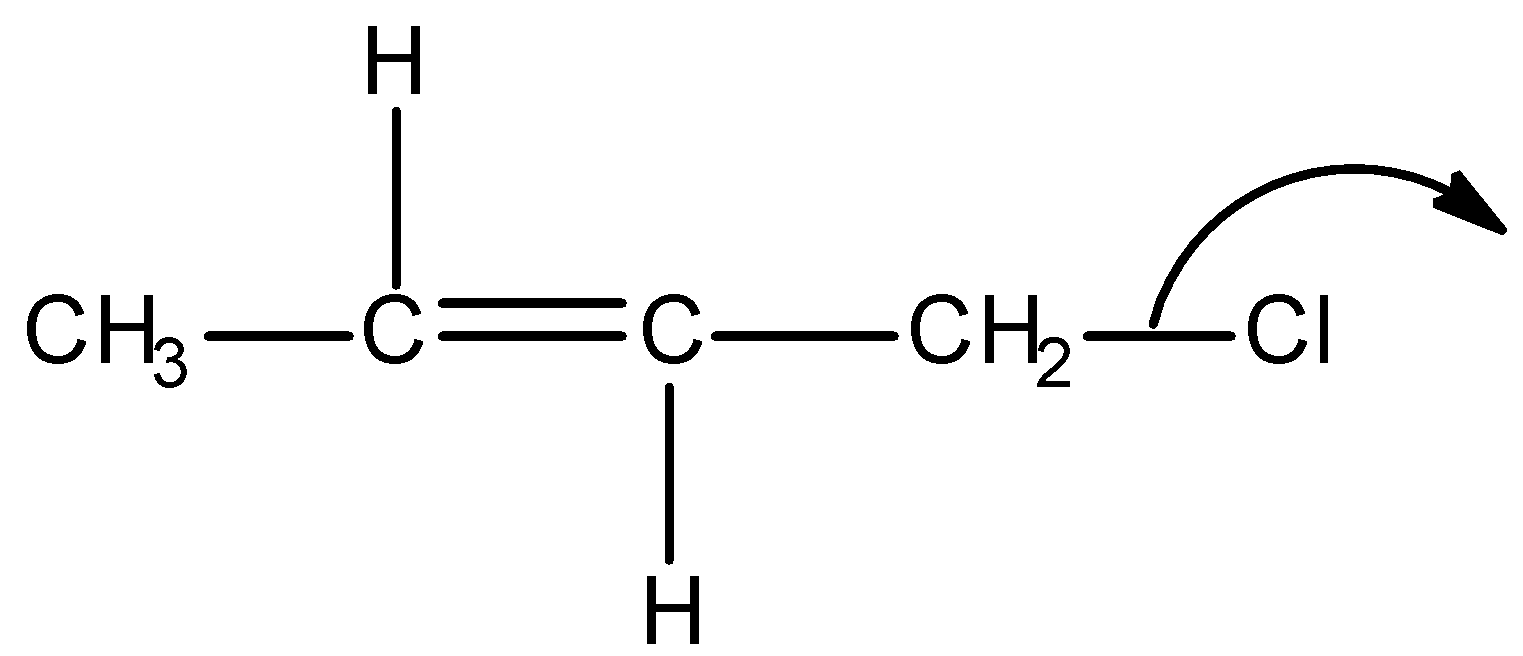
D)
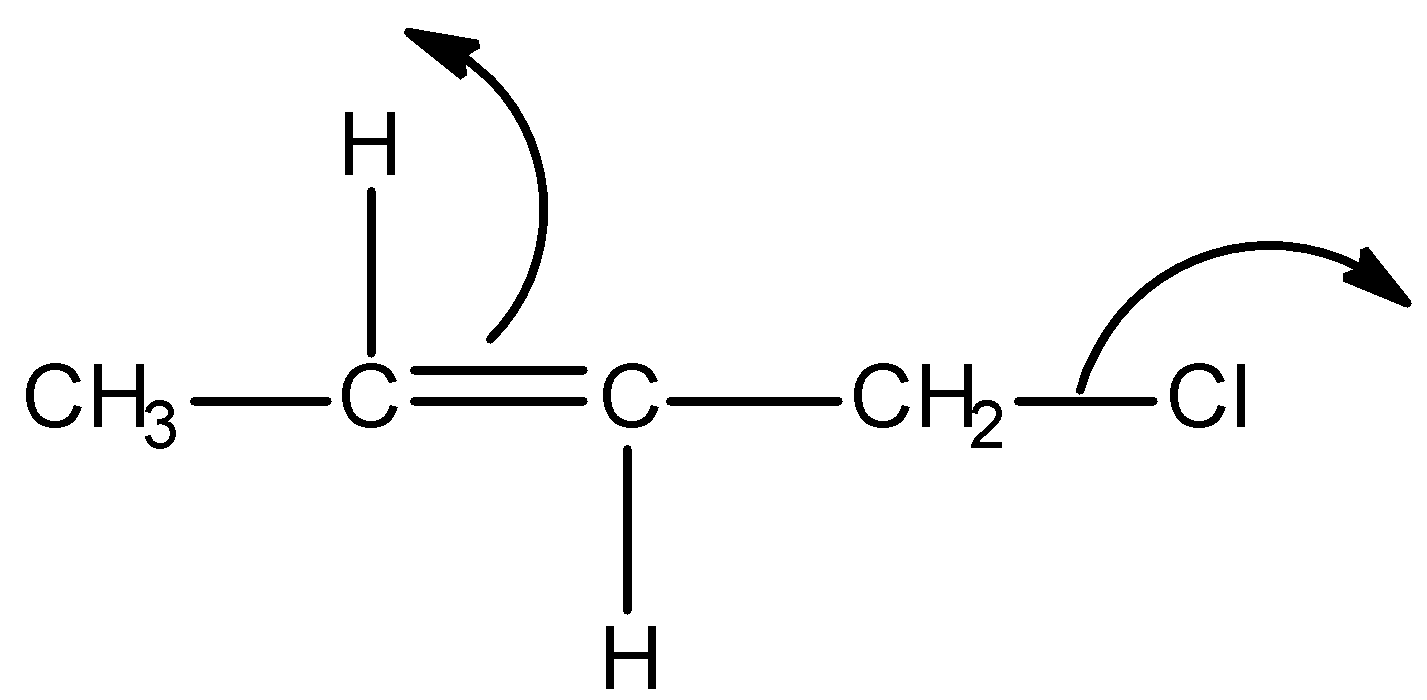
Solution
Refer to the mechanism of reverse hyperconjugation. Reverse hyperconjugation is most commonly observed in the case of α−Halo alkenes. In this phenomenon, there is movement of electron density from the filled π− or p-orbital to the neighbouring empty σ∗−orbital.
Complete answer:
Firstly, observe that we are given a particular type of system in all the options and it is as shown below:
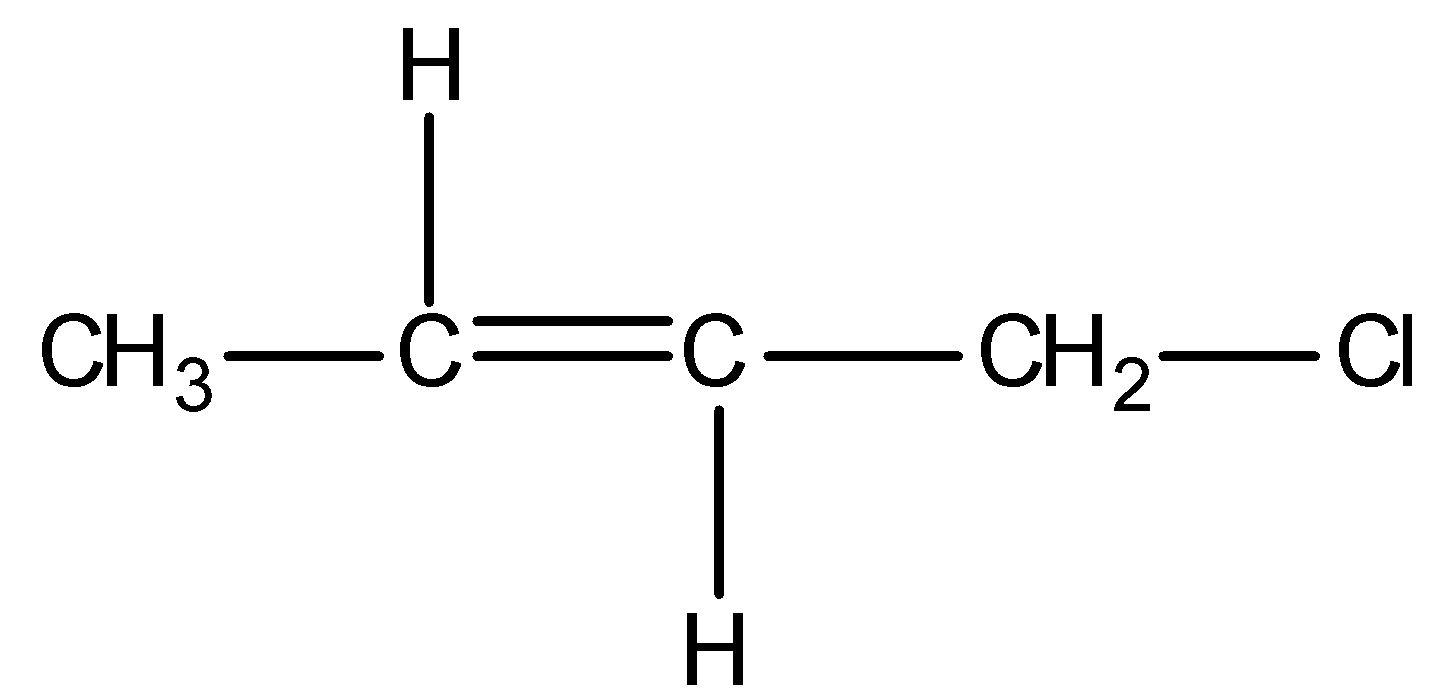
This above structure belongs to the class of α−Halo alkenes. Reverse hyperconjugation phenomenon is observed in α−Halo alkenes, where the σ∗−orbital is located on the carbon-halogen bond. In reverse hyperconjugation, there is movement of electron density from the filled π− or p-orbital to the neighbouring σ∗-orbital. Therefore, in case of α−Halo alkenes, there is delocalization of electron density from the double bond (i.e., filled π−orbital electron density) towards the electrophilic carbon (i.e., neighbouring σ∗-orbital). The electrophilic carbon or carbocation is generated when a halogen group leaves with its shared pair of electrons. Simply, we can say that delocalization of electrons occurs towards the halogen group through the hyperconjugative mechanism in α−Halo alkenes.
We can express the reverse hyperconjugation mechanism in the given α−Halo alkene as:
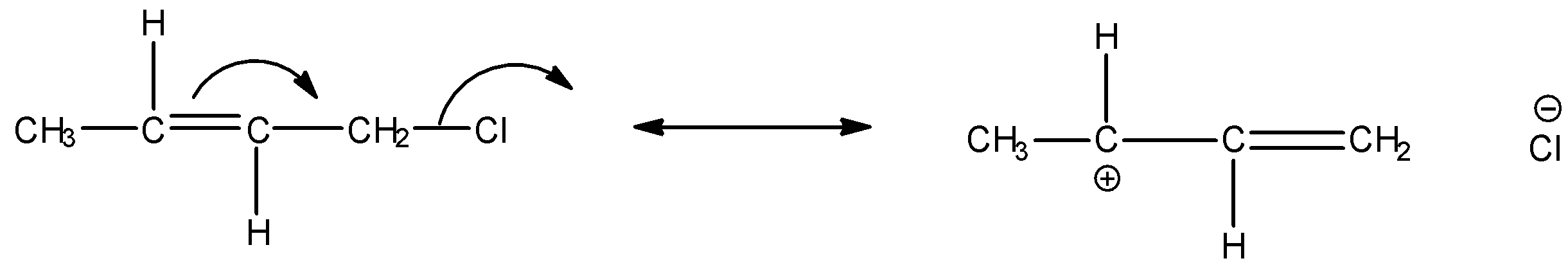
Thus among the given options, only in option A, reverse hyperconjugation mechanism is directed.
Hence, the correct option is A.
Note:
Reverse hyperconjugation phenomenon is also known as negative hyperconjugation. This is attributed to the fact that in negative hyperconjugation, the electron density flows in the opposite direction (from filled p-orbital to empty σ∗−orbital) than it does in the more common hyperconjugation phenomenon (from σ−orbital to empty π−orbital). Negative hyperconjugation stabilizes the molecule or transition state. It causes the elongation of the σ−bond by adding electron density to its anti-bonding orbital.
