Question
Question: Which of the following is the functional group of ether? A.
B.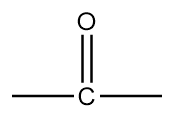
C. −O−
D. None of the above
Solution
Hint : A specific group of atoms or bonds within an organic compound which is responsible for the specific and characteristic chemical reactions of that compound is termed as a functional group. These groups are used to differentiate similar compounds from each other.
Complete Step By Step Answer:
A functional group in an organic compound plays a significant role in controlling and directing organic reactions. The presence of these groups allows for higher specificity and reactivity of an organic compound. Among given options, the given structures represent following functional groups:
Structure-A:
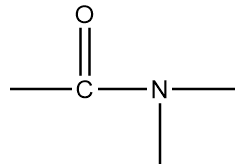
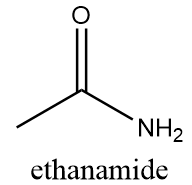
The name of this functional group is amide group in which a carbonyl group is covalently bonded to azide ion (NH2−) or its derivative (NR2−). An example of a compound containing amide group is as follows:
Structure-B:
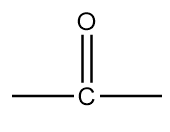
The name of this functional group is ketone in which a carbonyl group is covalently bonded with two alkyl groups which can either be same or different. An example of a compound containing ketone functional group is as follows:
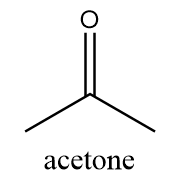
Structure-C: −O−
The name of this functional group is ether in which an oxygen atom is bonded to two alkyl groups via a single bond. The alkyl groups can either be the same or different. Because of the low reactivity of ethers, these are widely used as solvents in many organic reactions. An example of a compound containing an ether as a functional group is as follows:
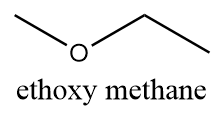
Hence, the functional group which represents ether is −O−. Thus, option (C) is the correct answer.
Note :
Make sure to not to get confused between the ether and ester functional group. Although the name is similar but in ether, the oxygen atom is directly bonded to alkyl groups whereas in ester, a carbonyl group is also present within the structure.
