Question
Question: Which of the following is the functional group of ketones. (A) 
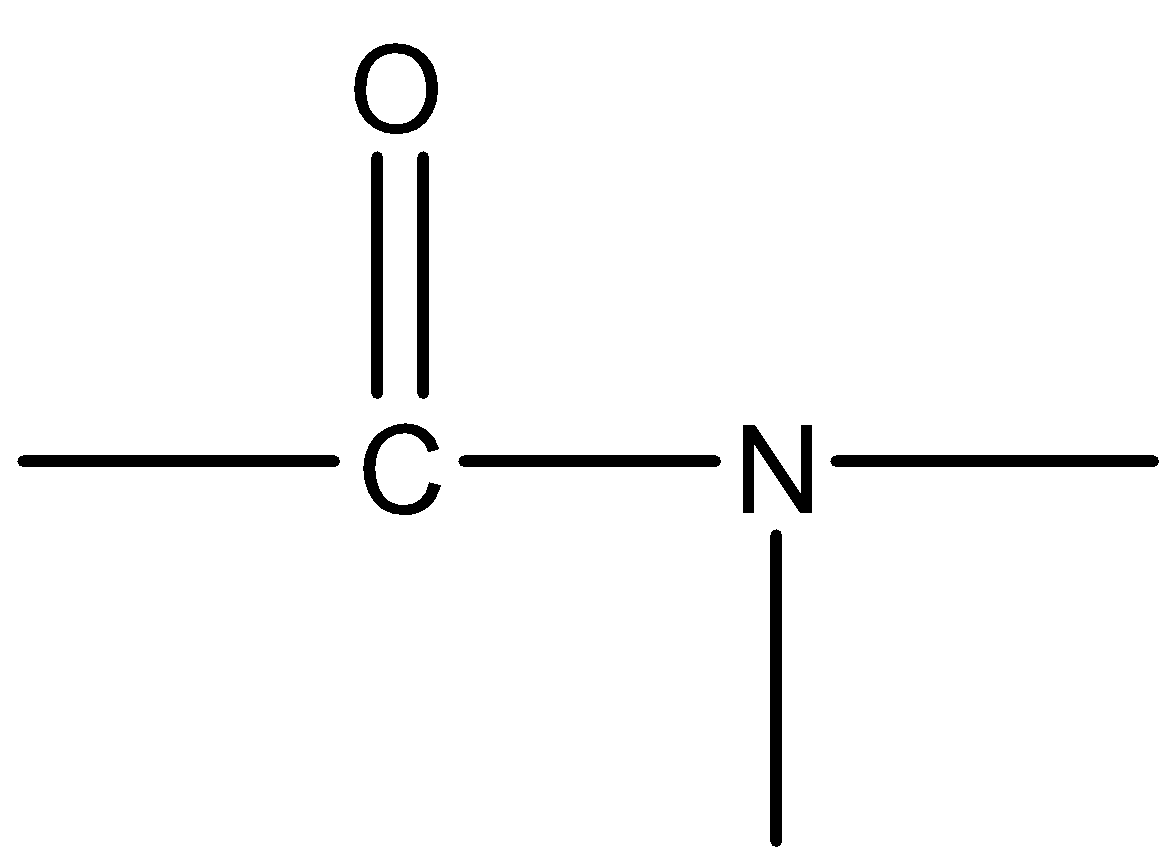
(B)
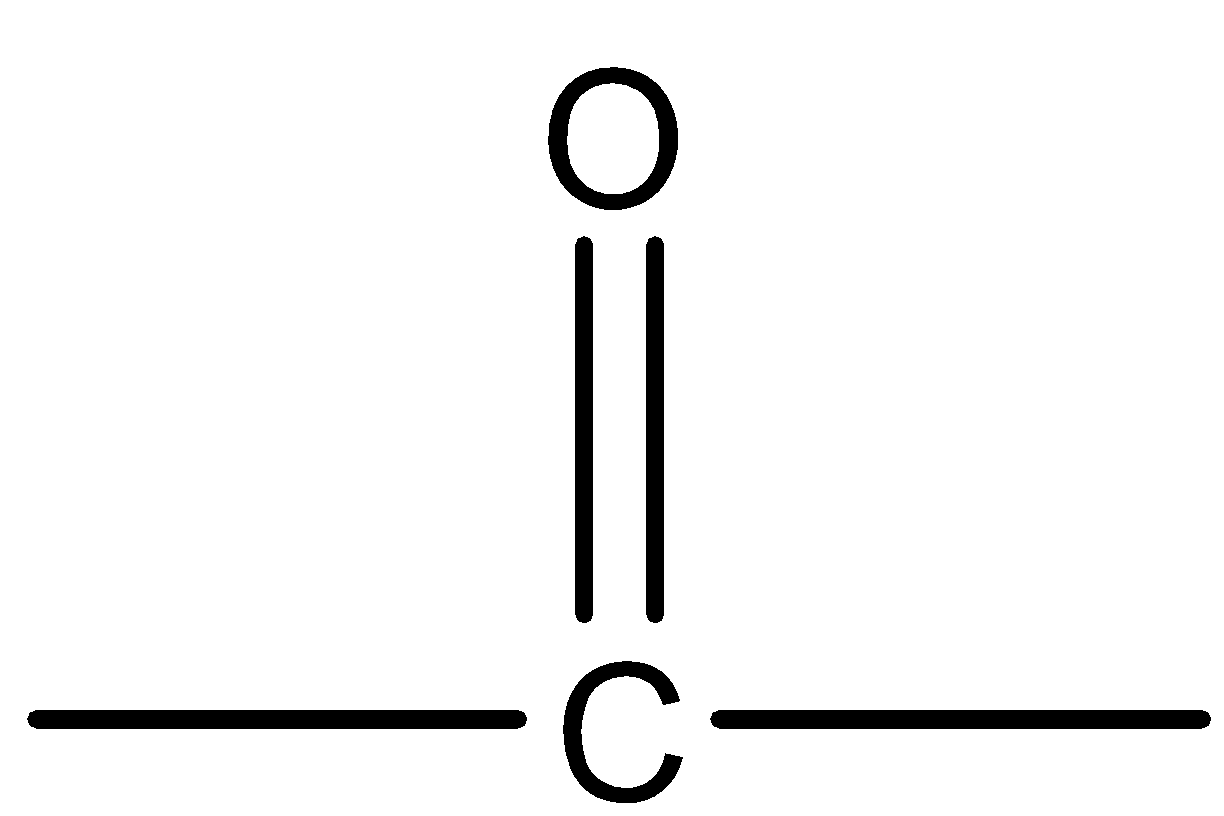
(C)
(D) None of these.
Solution
Functional groups in organic chemistry are particular species or substituents in the molecules that are responsible for the properties of that molecule. The atoms present in the functional groups are bonded to each other and with the molecule via covalent bonding.
Complete Step-by-step Answer:
The chemical and physical properties of a compound are determined by the functional group that is attached to the molecule. The interconversion of one functional group to another can be done in organic chemistry via different substitution reactions. There are 14 main functional groups in organic chemistry and 8 side functional groups. They are more like side substituents in the molecule. Some examples of functional groups are Aldehydes, ketones, Amines, Alcohols, Carboxylic acid, Ester, etc.
Ketones are one of the main functional groups that have a sp2 hybridized carbonyl carbon at which carbon substituents are attached on both sides. The structure is trigonal planar with a bond length of 1200 . The functional group of ketone is 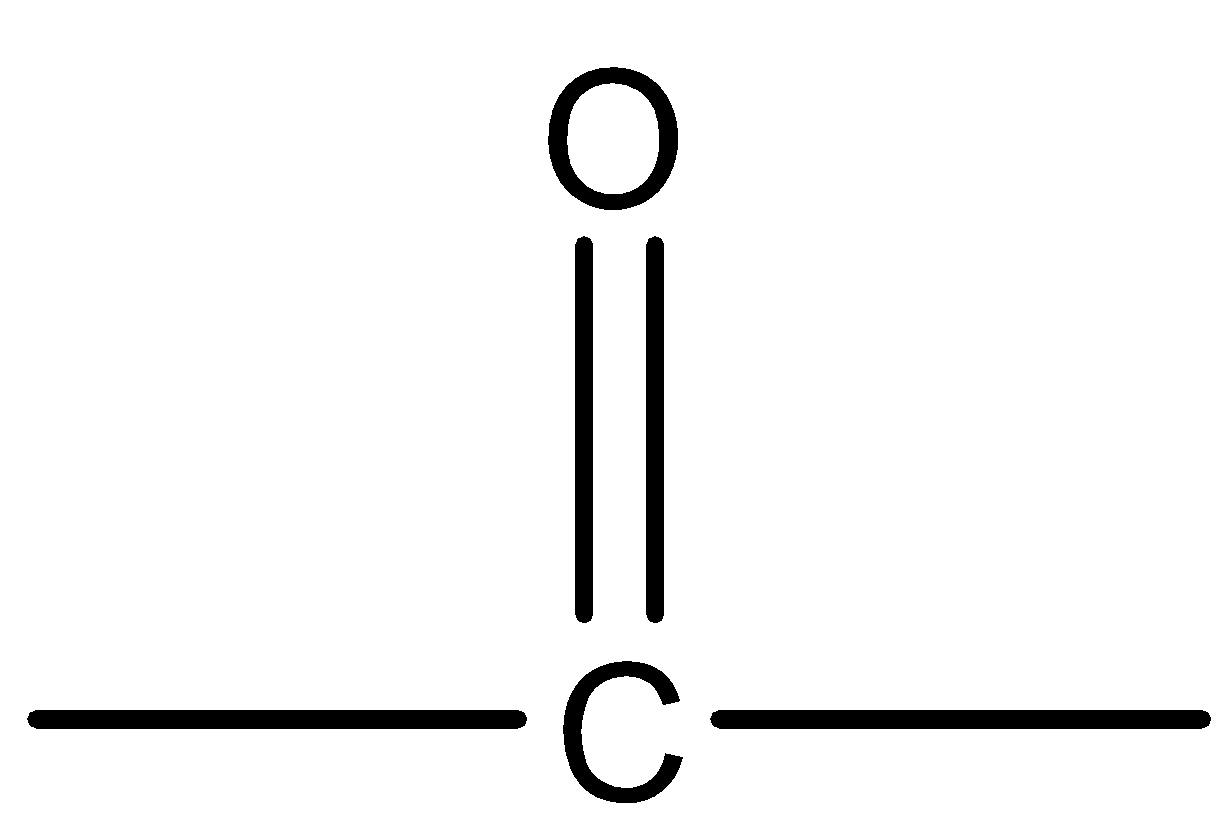 and the general structure of ketones is
and the general structure of ketones is 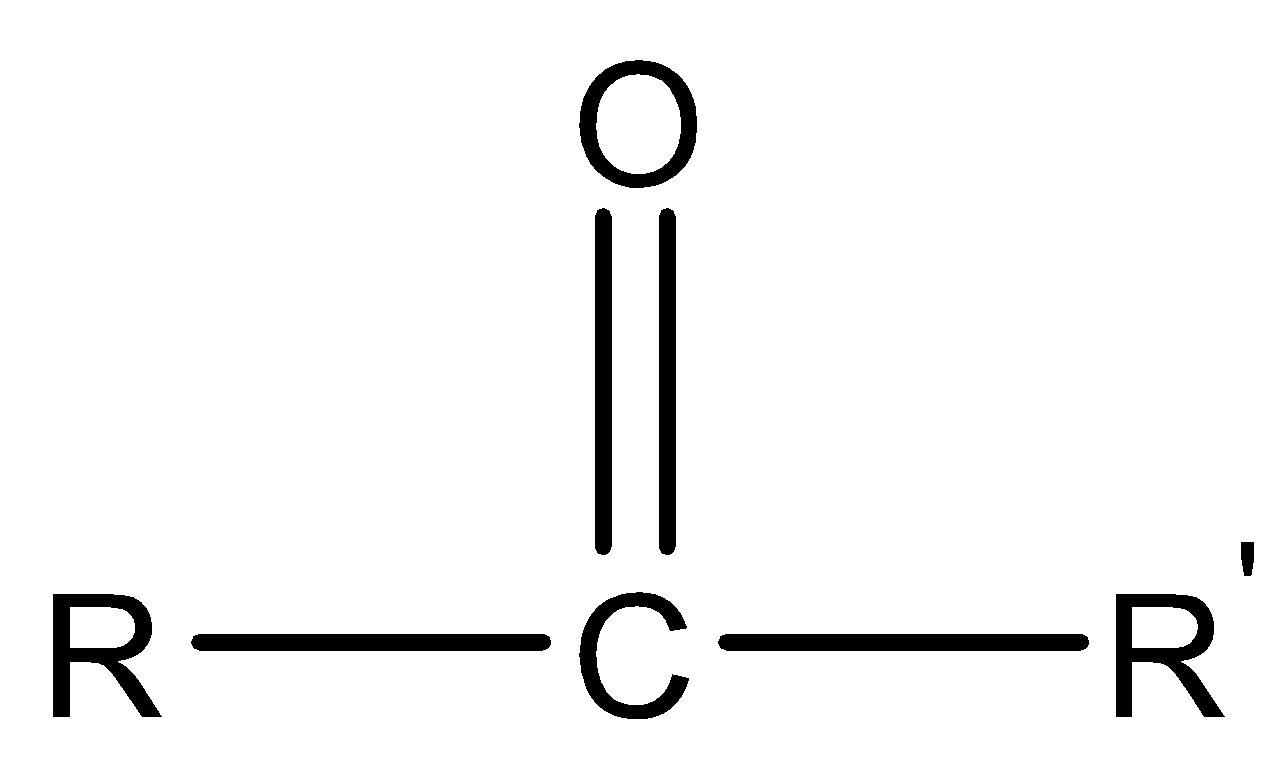 . Due to the presence of oxygen atoms with carbon the −CO molecule tends to be polar. Due to the polar nature, they have a high boiling point. The dipole moment of ketones is also higher than alcohol as no hydrogen atom is attached with oxygen. The naming of ketones is done by adding the suffix “ anone” after the parent chain. The position of the carbonyl group is represented by a number.
. Due to the presence of oxygen atoms with carbon the −CO molecule tends to be polar. Due to the polar nature, they have a high boiling point. The dipole moment of ketones is also higher than alcohol as no hydrogen atom is attached with oxygen. The naming of ketones is done by adding the suffix “ anone” after the parent chain. The position of the carbonyl group is represented by a number.
Hence, we can say that 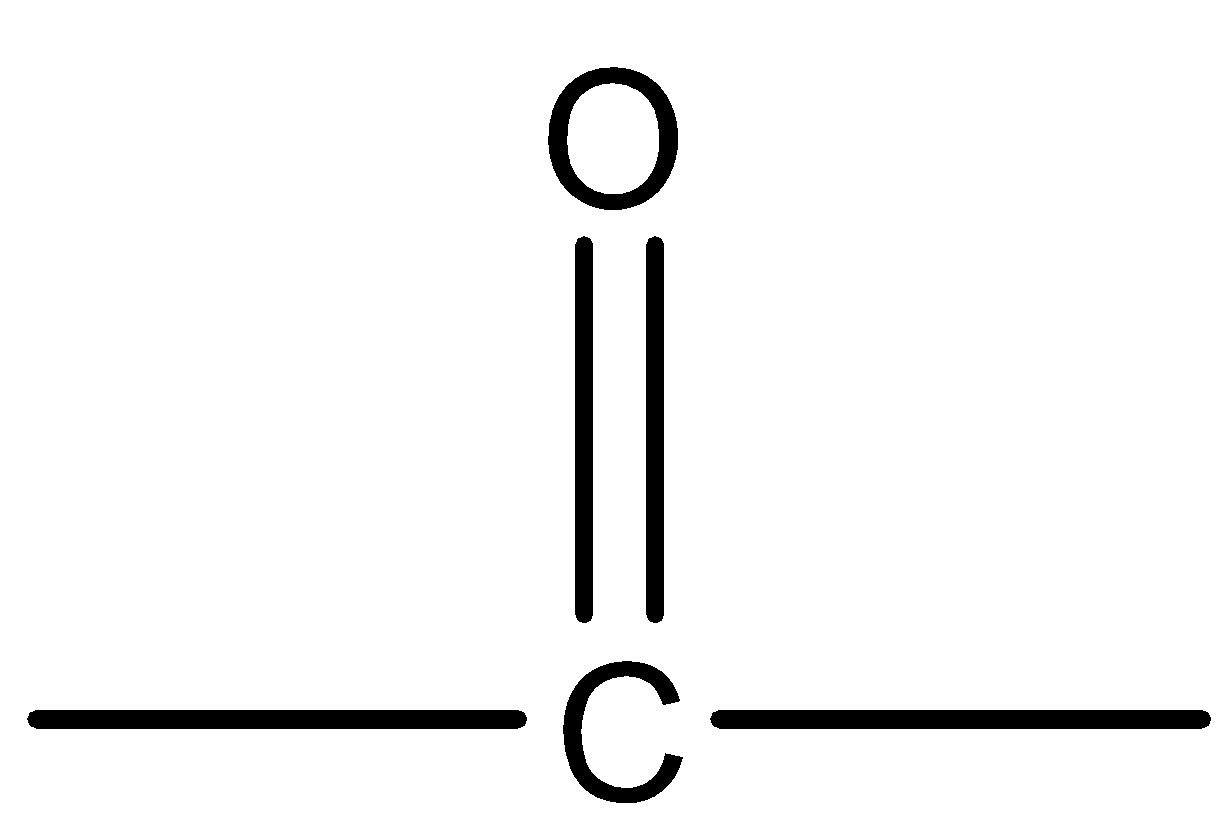 is the functional group of ketones.
is the functional group of ketones.
Therefore, option (B) is correct.
Note:
Ketones are mostly used in the making of solvents due to their polar nature. They are also used in the household as thinner which is acetone. In the medical field, they are used in chemical peeling and acne treatment.
