Question
Question: Which of the following is the correct statement regarding the following four alkyl halides (I to IV)...
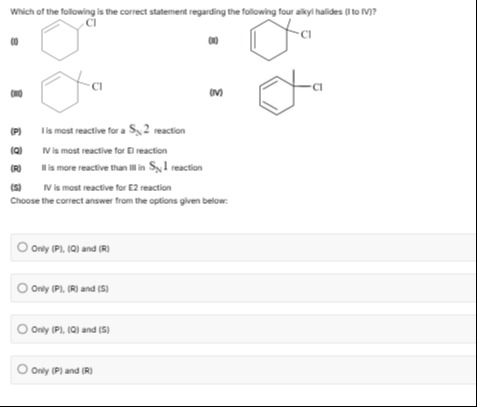
Which of the following is the correct statement regarding the following four alkyl halides (I to IV)?
(P) I is most reactive for a SN2 reaction
(Q) IV is most reactive for E1 reaction
(R) II is more reactive than III in SN1 reaction
(S) IV is most reactive for E2 reaction
Choose the correct answer from the options given below:
I is most reactive for a SN2 reaction
IV is most reactive for E1 reaction
II is more reactive than III in SN1 reaction
IV is most reactive for E2 reaction
All statements (P, Q, R, and S) are correct. However, the provided options do not reflect this.
Solution
Let's analyze each alkyl halide and its reactivity for SN1, SN2, E1, and E2 reactions.
Structures:
- I: Cyclohex-2-enyl chloride (3-chlorocyclohex-1-ene). The carbon bearing Cl is a secondary allylic carbon.
- II: 1-chloro-1-methylcyclohex-2-ene. The carbon bearing Cl is a tertiary allylic carbon.
- III: 1-chloro-1-methylcyclohexane. The carbon bearing Cl is a tertiary alkyl carbon.
- IV: 1-chloro-1-methylcyclohexa-2,4-diene. The carbon bearing Cl is a tertiary allylic carbon with extended conjugation (two adjacent double bonds).
Analysis of Statements:
(P) I is most reactive for a SN2 reaction.
SN2 reactivity is primarily governed by steric hindrance at the carbon bearing the leaving group. The general order is Methyl > Primary > Secondary > Tertiary. Since I is a secondary halide, it experiences less steric hindrance compared to the tertiary halides (II, III, IV). Tertiary halides are generally very unreactive towards SN2 due to significant steric hindrance. Therefore, I will be the most reactive for SN2. Statement (P) is correct.
(Q) IV is most reactive for E1 reaction.
E1 reaction proceeds via a carbocation intermediate, so its reactivity depends on the stability of the carbocation formed. The stability order of carbocations formed from these halides is: IV (Tertiary allylic with extended conjugation) > II (Tertiary allylic) > III (Tertiary alkyl) > I (Secondary allylic). Since IV forms the most stable carbocation, it will be the most reactive for E1 reaction. Statement (Q) is correct.
(R) II is more reactive than III in SN1 reaction.
SN1 reactivity depends on carbocation stability. A tertiary allylic carbocation (from II) is stabilized by both resonance and hyperconjugation, making it more stable than a simple tertiary alkyl carbocation (from III), which is primarily stabilized by hyperconjugation. Therefore, the carbocation from II is more stable than the carbocation from III, and thus II is more reactive than III in SN1 reaction. Statement (R) is correct.
(S) IV is most reactive for E2 reaction.
E2 reaction depends on the stability of the product alkene. Elimination of HCl from IV leads to the formation of 1-methylbenzene (toluene). The formation of an aromatic ring is highly favorable due to the immense stabilization energy of aromaticity. The formation of an aromatic compound provides a significantly greater driving force for elimination than the formation of conjugated dienes or simple substituted alkenes. Therefore, IV will be exceptionally reactive for E2 elimination. Statement (S) is correct.
All statements (P), (Q), (R), and (S) are correct. The question is flawed because there is no option indicating that all four statements are correct.
