Question
Question: Which of the following is the correct representation when \[B{F_3}\] reacts with ammonia? 1) 
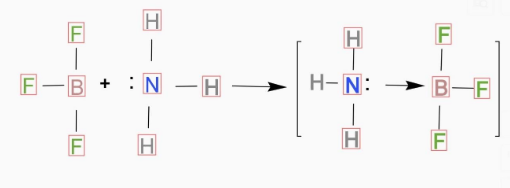
(A) 1 is incorrect and 2 is correct
(B) 1 is correct and 2 is incorrect
(C) Both 1 and 2 are correct
(D) Both 1 and 2 are incorrect
Solution
To answer the question we must know about the concept of Lewis acid and Lewis base. Both BF3and NH3 are Lewis acid and Lewis base respectively. And we have to know the octet rule and the concept of stable compounds. A detailed discussion is shown below.
Complete step-by-step answer: Lewis acid: In the Lewis hypothesis of acid-base reactions, bases give sets of electrons and acids acknowledge sets of electrons. A Lewis acid is any substance that can acknowledge a couple of nonbonding electrons. As such, a Lewis acid is an electron-pair acceptor.
One favorable position of the Lewis hypothesis is the manner in which it supplements the model of oxidation-reduction reactions. Oxidation-reduction reactions include an exchange of electrons starting with one particle then onto the next, with a net change in the oxidation number of at least one atom.
Lewis base: A Lewis base is a substance which can donate at least a pair of nonbonding electrons. That means it can be called an electron pair donor.
In the above question, two molecules are NH3andBF3. In NH3there are 5 electrons in the outer shell of N and 3electrons are involved in the bond formation with H atom and rest of the electrons remains as lone pair that means nonbonding electron-pair. So, we can conclude NH3 is a Lewis base.
And in BF3 there are 3 electrons in the outer shell of Band all are involved in bond formation so there is no nonbonding electron left in BF3and we can consider BF3 as a Lewis acid.
So the correct representation for the reaction of BF3 with NH3 will be:
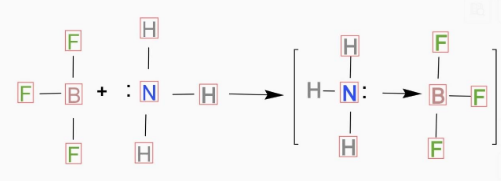
Hence the correct option is (A).
Note: In the reaction it is basically an acid base reaction. When BF3 reacts with NH3 an adduct is formed shown in the above diagram. And in that adduct N will donate electrons to B centre.
