Question
Question: Which of the following is the correct representation of the electron dot structure of nitrogen A....
Which of the following is the correct representation of the electron dot structure of nitrogen
A.
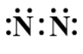
B.
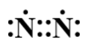
C.
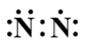
D.

Solution
To answer this question, you should recall the concept of drawing Lewis structures. A Lewis structure of a chemical species is a graphic representation of the electron distribution around the constituent atoms. The number of electron pairs shared represents the number of bonds.
Complete Step by step solution:
Lewis structures are used to predict the number and type of bonds that may be formed around an atom. The steps that must be followed while drawing a Lewis structure are:
Calculation of total valence electrons present in the molecule which can be obtained by adding the individual valencies of each atom.
In the case of anion, the extra electrons are added to the Lewis dot structure. While in case of cation electrons are subtracted from the total valence electron count.
The least electronegative atom is made the central atom of the overall molecule and the peripheral atoms are now connected via single bonds.
Assign lone pair electrons staring from the most electronegative atoms first.
Draw double or triple bonds for the central atom to satisfy the octet valency of each atom.
Using the above rules in a nitrogen molecule, each nitrogen atom has 5 valence electrons and there is a triple bond between the two nitrogen atoms.
Thus, the correct electron dot structure is:

Thus, the correct answer to this question is option D.
Note Bond length in a molecule is defined as the distance between the centres of the two covalently bonded atoms.
The length of the bond is determined by the number of bonded electrons (the bond order). If bond order is high, there will be a stronger pull between two atoms and there will be shorter bond length. Therefore, bond length increases in the following order: triple bond < double bond < single bond.
