Question
Question: Which of the following is the correct order of stability of free radicals? A) Benzyl > allyl > \[2...
Which of the following is the correct order of stability of free radicals?
A) Benzyl > allyl > 2∘ > 1∘
B) Allyl > benzyl > 2∘ > 1∘
C) Allyl > 2∘ > 1∘ > benzyl
D) benzyl > 2∘ > 1∘ > allyl
Solution
We must have to know that in organic chemistry stability of molecules and ions are very important. Carbon ions are classified as two types. There is carbocation and carbanion. The carbon atom which has a positive charge is called carbocation. The carbon atom which has negative charge is called carbocation. In organic chemistry cleavage is split into two types. There are homolytic cleavage and heterolytic cleavage. Ions will happen in heterolytic cleavage. Free radicals will come in Homolytic cleavage.
Complete answer:
The given free radicals are
Benzyl, allyl,1∘, 2∘
The primary free radical representation is 1∘.
The secondary free radical representation is 2∘.
The structure of benzyl free radicals is
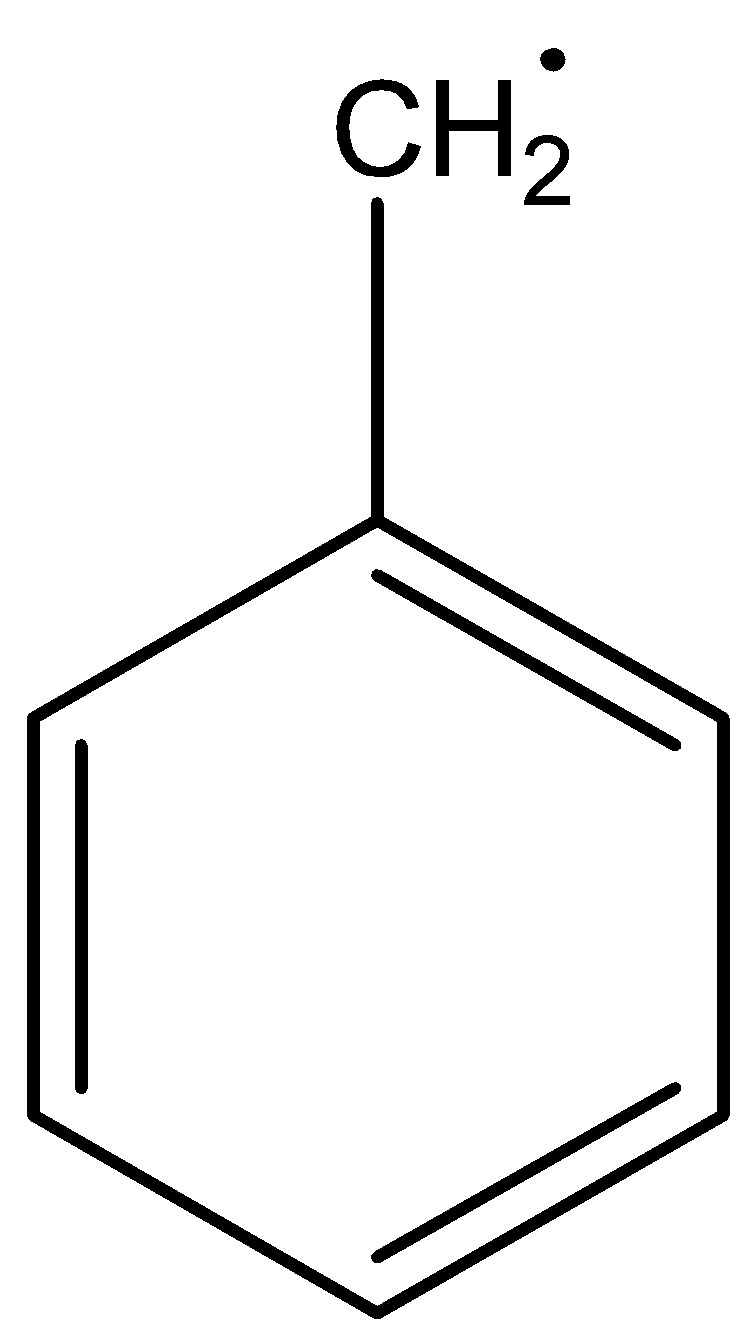
The structure of allyl free radicals is
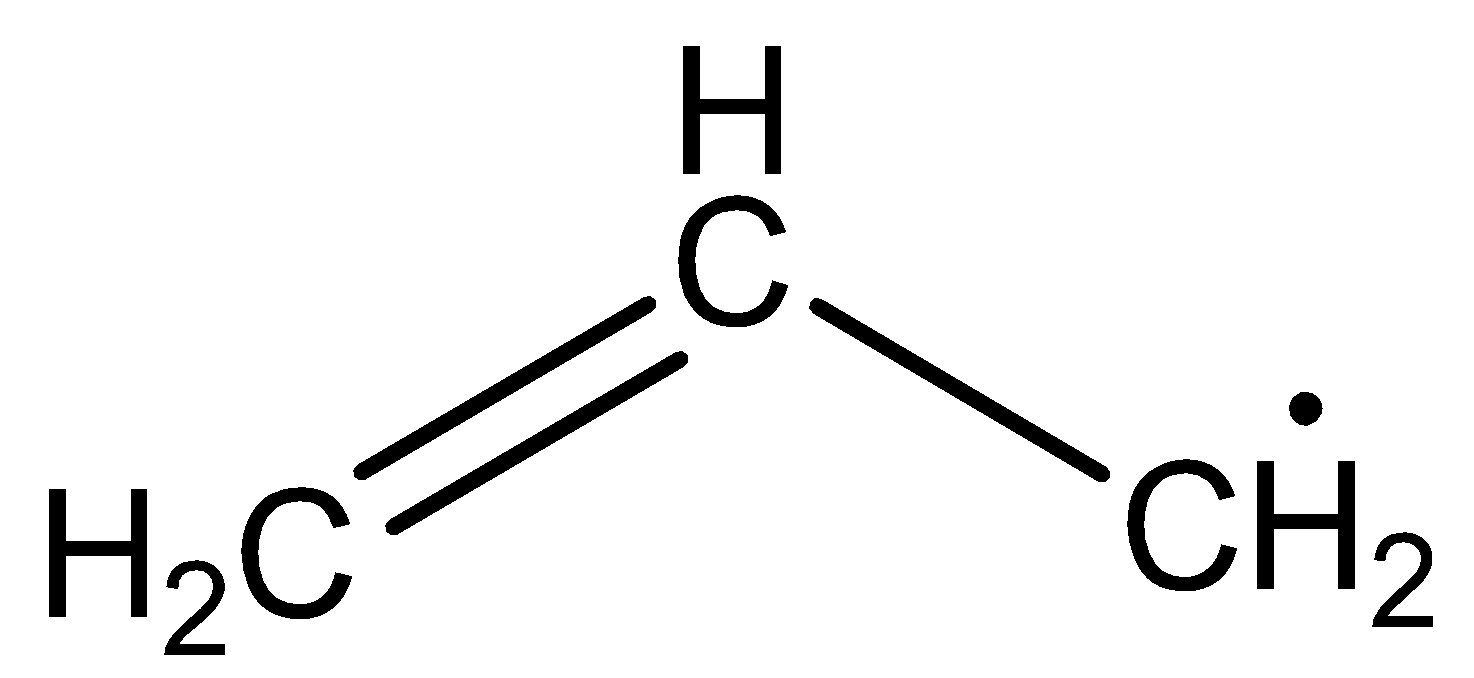
The structure of primary free radicals is
CH3.
The structure of secondary free radicals is
(CH3)2CH.
Benzyl free radicals are most stable in the given series.
Primary free radicals are most stable in the given series.
According to the above discussion, we conclude the order of stability of free radicals is
Benzyl > allyl > 2∘ > 1∘
Hence, option A is the correct answer.
Note:
We must have to remember that benzyl free radicals are more stable than primary free radicals. Benzyl free radicals are more stable than allyl free radicals. Benzyl free radicals are more stable than secondary free radicals. Allyl free radicals are more stable than secondary free radicals. Allyl free radicals are more stable than primary free radicals. Secondary free radicals are more stable than primary free radicals.
