Question
Question: Which of the following is the correct answer? 
A.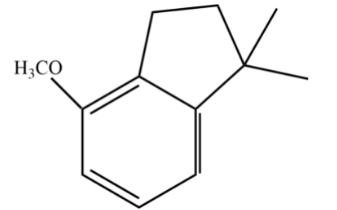
B.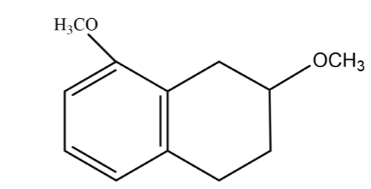
C.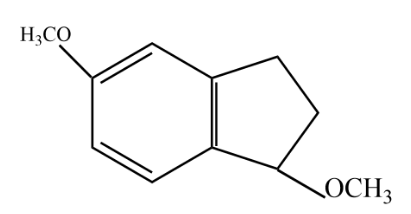
D.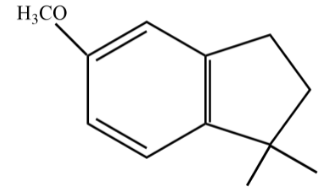
Solution
Friedel crafts alkylation reaction is an electrophilic aromatic reaction in which a carbocation attacks an aromatic ring with the end result that one of the aromatic protons is replaced by an alkyl group.
Complete answer:
If we prefer, we can regard these reactions as involving an attack by an aromatic ring on a carbocation. This latter approach is used in the textbook, but the former approach is probably common. When more than one alkyl group is introduced into an aromatic ring during the course of a Friedel crafts alkylation reaction, polyalkylation is said to have occurred.
Step 1: The Cl reacts with AlCl2, which makes the CHCl2 bond weak.
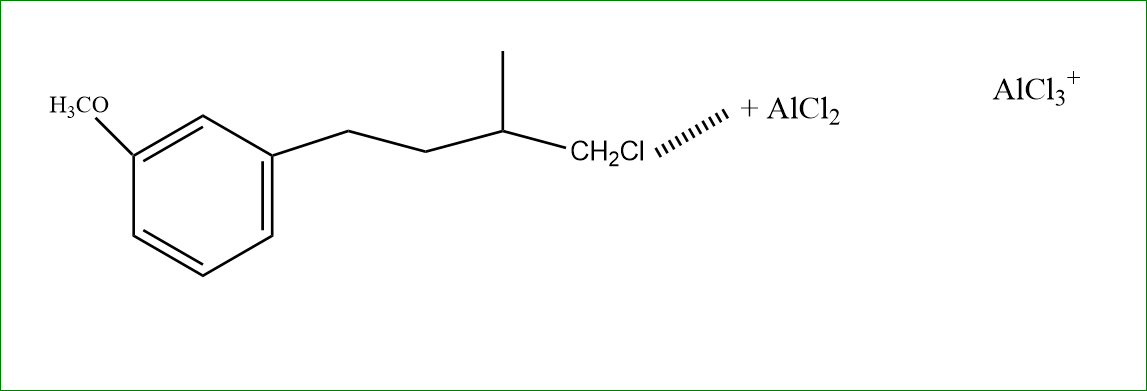
Now in step 2: There is a formation of carbocation.
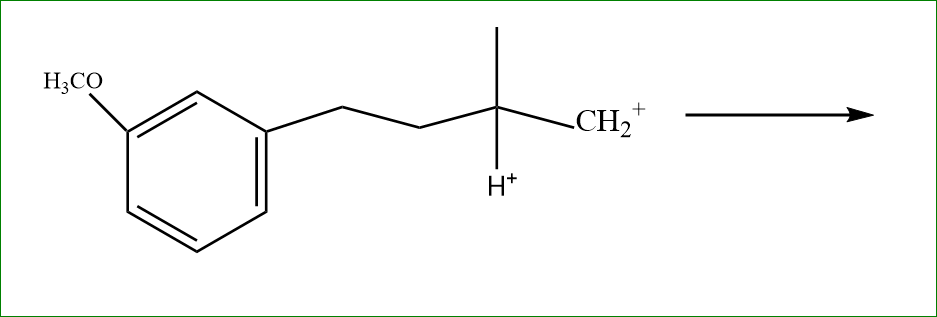
We know that more stable carbocation is tertiary, So the carbocation moves to tertiary carbon with eliminating the hydrogen bond.
Step 3: If there are more than 3, they try to form rings if possible. Hence the tertiary carbocation vacancy is filled by extracting electrons from the nearest Pi bond.
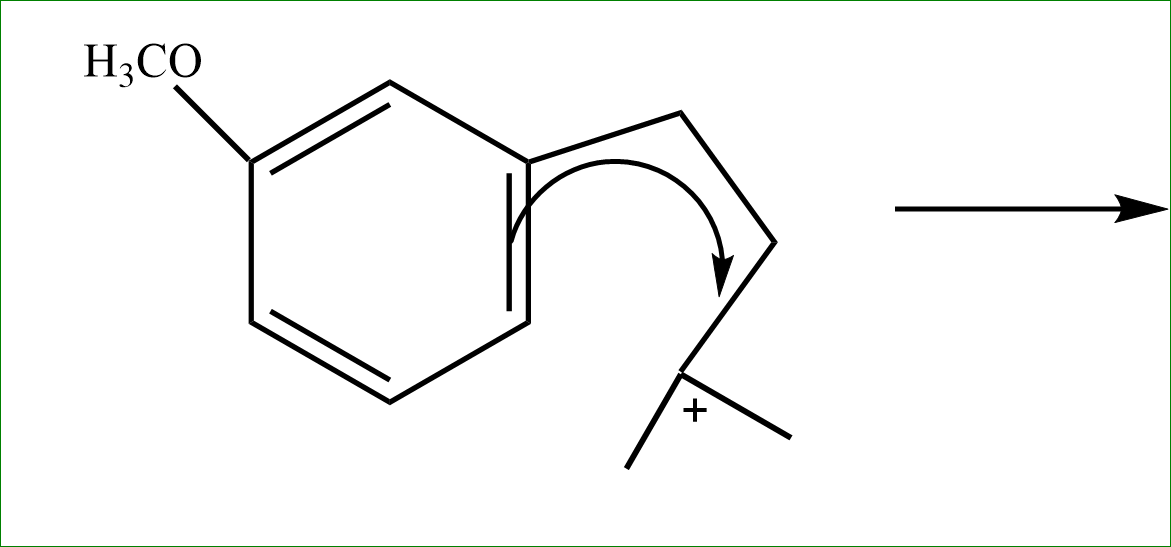
Step 4: the adjacent hydrogen is released further, to satisfy the valency and the bond is formed on the penta ring cycle side. Here intramolecular alkylation occurs.
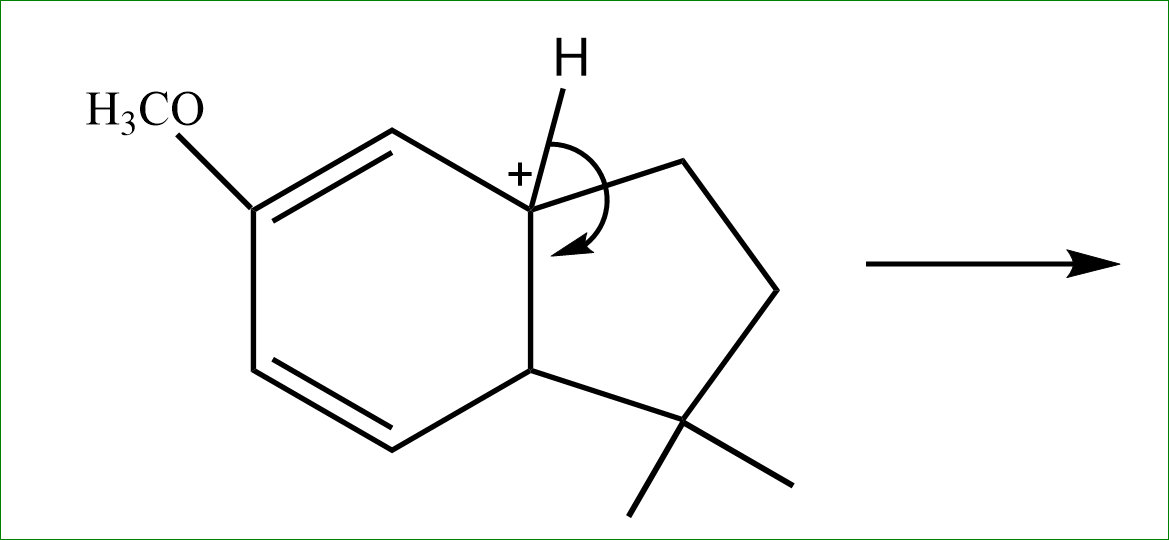
So, the answer is option (D).
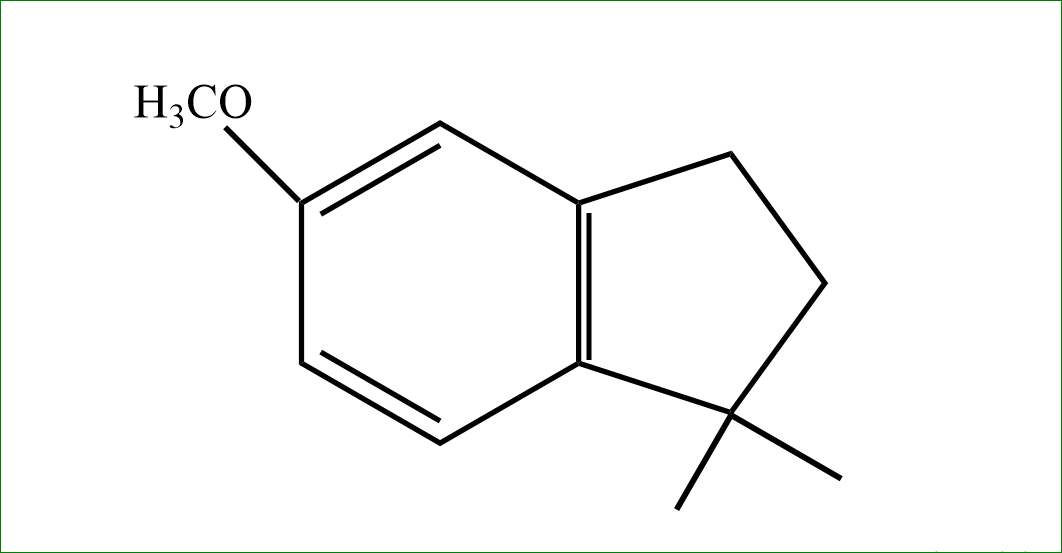
Note:
The reaction of an aromatic substrate with an acid chloride in the presence of an aluminium chloride catalyst is used to introduce an acyl group into the aromatic ring through an electrophilic aromatic substitution mechanism.
