Question
Question: Which of the following is the best reagent to convert 1-methylcyclohexene into 2-methylcyclohexanol?...
Which of the following is the best reagent to convert 1-methylcyclohexene into 2-methylcyclohexanol?
A. Dil. H2SO4
B. Hg(OAc)2/NaBH4,H2O
C. B2H6/H2O2,OH−
D. Conc.H2SO4
Solution
To convert to convert 1-methylcyclohexene into 2-methylcyclohexanol, we need to add H2O on 1-methylcyclohexene according anti markovnikov law. That can be done by making it react with diborane, that will form a trialkyl borane with it and then oxidizing the trialkyl borane into the alcohol.
Complete step by step answer:
Diborane (B2H6) is the dimer of BH3. BH3is a colorless gas. In this molecule Boron has an empty orbital and that’s why it forms dimer with another BH3 molecule via banana bonding to form its dimer.
Anti Markovnikov rule: According to this rule when electrophilic addition of a compound takes place on alkene then electrophile gets attached on the more substituted carbon atom.
Electronegativity of hydrogen is more than boron, that’s why whenBH3 reacts, it releases hydrogen as hydride ion. So on reacting with given cycloalkene, addition of hydride ion and boron will take place according to markovnikov rule and trialkyl borane forms.
Now when this trialkyl borane gets oxidized by H2O2 in alkaline medium, in place of each boron, one hydroxide ion forms a bond with carbon and a cycloalkane forms.
Overall addition of water is taking place on 1-methylcyclohexene where hydrogen is getting attached to more substituted carbon while hydroxide ion is getting attached on less substituted carbon.
Therefore overall it is similar to anti markovnikov addition.
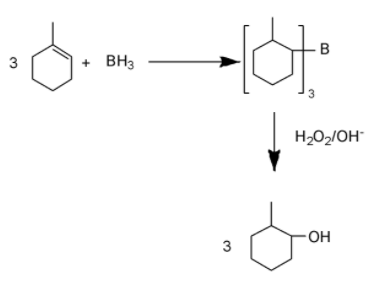
So, the answer is option C.
Additional information:
According to markovnikov rule when electrophile attacks on alkene, it gets attached to the less substituted carbon atom.
Note: 2-methylcyclohexanol is a colorless liquid with boiling point of 163−1660C. It is slightly soluble in water and has specific gravity equal to 0.930. 1-methylcyclohexene is a colorless clear liquid with boiling point of110−1110C. It is soluble in benzene and ether and miscible with many hydrocarbons of similar structure.
