Question
Question: Which of the following is reduced with Zn-Hg and HCl to give alkane? (a).Ethyl acetate (b).Aceti...
Which of the following is reduced with Zn-Hg and HCl to give alkane?
(a).Ethyl acetate
(b).Acetic acid
(c).Acetamide
(d).Butan-2-one
Solution
The reagent given in this question is used for clemmensen reduction. It results in deoxygenation of aldehydes or ketones.
Complete answer:
In organic chemistry, reagents play the most important part in any reaction.
Zn-Hg in acidic medium (conc. HCl) is the reagent for clemmensen reduction.
The mechanism for clemmensen reduction is given as –
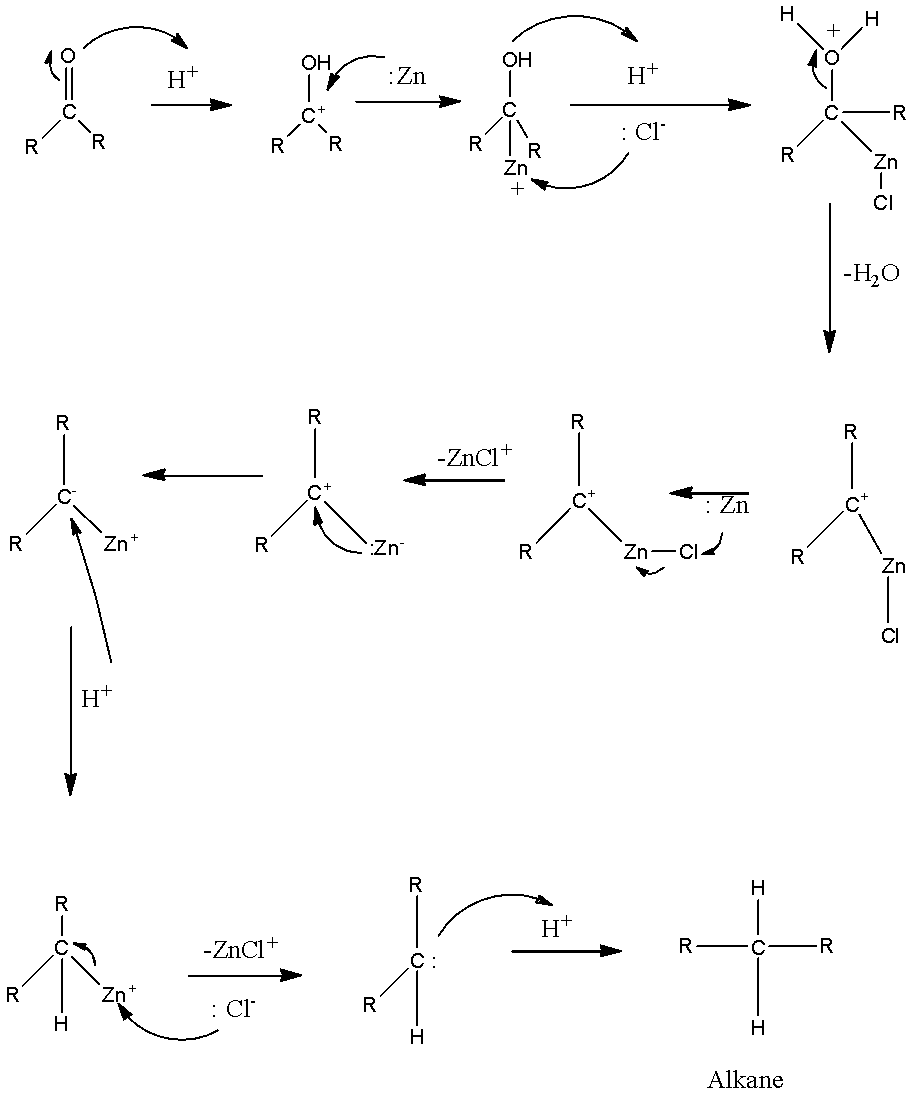
Amongst the given options,
Butan-2-one is a ketone
Acetamide is an amide
Acetic acid is an acid
Ethyl acetate is an acetate
Clemmenson reagent acts only on aldehydes and ketones. Therefore, butan-2-one will get reduced to butane when we treat it with zinc and hydrochloric acid.
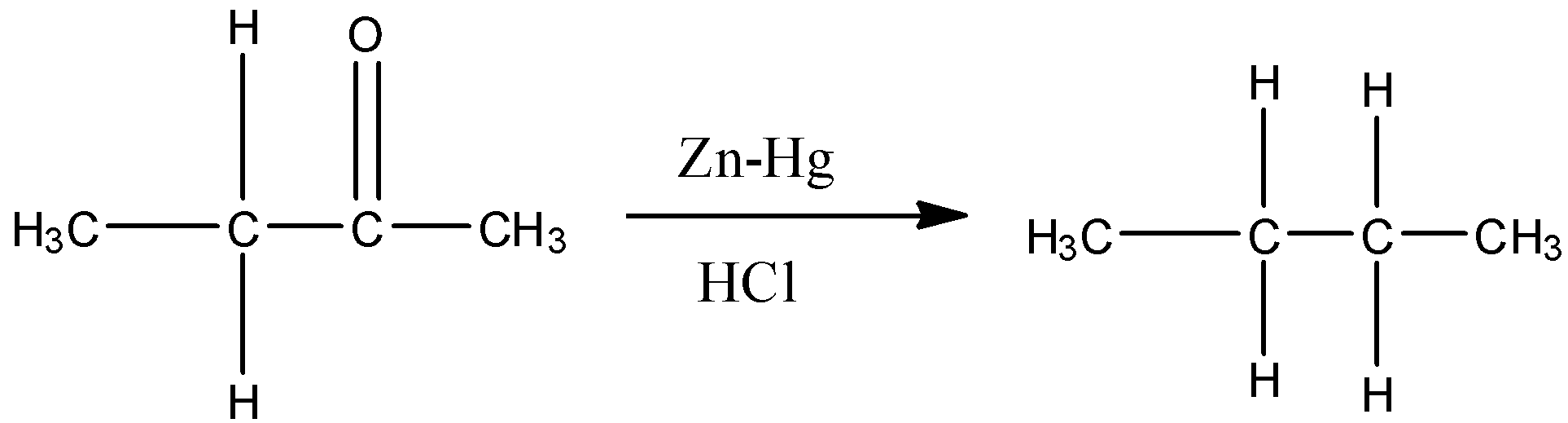
Therefore, the answer is – option (a) – Butan-2-one is reduced with Zn-Hg and HCl to give alkane.
Note:
Wolf Kishner reduction is also a method for the preparation of alkanes from aldehydes and ketones. The reagent used in this reaction is hydrazine - in a basic solvent like glycol.
