Question
Question: Which of the following is polar? A.\({\text{N}}{{\text{F}}_{\text{3}}}\) B.\({\text{B}}{{\text{F...
Which of the following is polar?
A.NF3
B.BF3
C.SF4
D.SiF4
Solution
A polar molecule is formed when the one end of the molecule is said to have a high affinity towards the shared pairs of electrons and thus attracts those electrons towards itself and thus a partial negative charge.
Since the electrons are pulled away from the other end of the molecule it attains a partial positive charge and this difference of polarity between the two ends of the molecule gives rise to a non-zero dipole moment and the molecule is said to be polar.
This polarity arises from a difference in the electro-negativities of the bonded atoms.
Complete step by step answer:
To predict which one the given molecule is polar let us look into the structure of each one on these:
NF3:
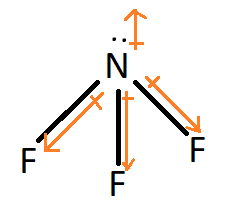
Fluorine is more electronegative than nitrogen and thus attracts the shared pair of electrons towards itself. Nitrogen has a lone pair of electrons and therefore the geometry NF3 is not exactly trigonal planar but slightly distorted due to lone pair-bond pair repulsion and thus the bond angle is less than 120 degrees. This results in a non-zero dipole moment.
Therefore, NF3 is a polar molecule.
BF3:
There is a difference in the electronegativities of the Boron and Fluorine but they are arranged in trigonal planar and thus cancel out each other’s effect:
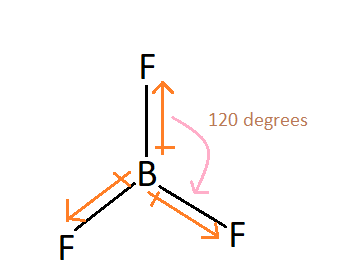
Thus the resultant dipole moment zero and BF3 is a nonpolar molecule.
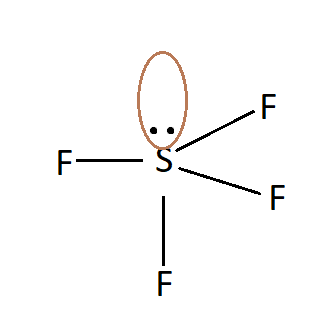
SF4:
It has a trigonal bipyramidal geometry, having 4 bond pairs and 1 lone pair. Due to the presence of lone pair, there is a net non-zero dipole moment and thus SF4 is a polar molecule.
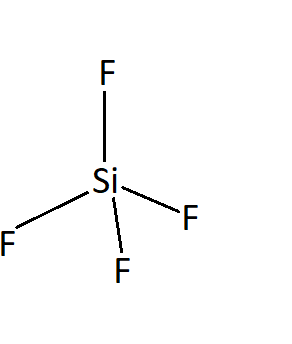
SiF4:
The bond between silicon and fluorine is polar due to the electronegativity difference but since the geometry of SiF4 is perfectly tetrahedral, the effect of all the bonds is cancelled out and there is no net dipole moment. Thus, SiF4 is a nonpolar molecule.
Thus, it can be concluded that NF3 and SF4 are polar molecules.
Therefore, the correct answer is A and C.
Note: Other than polar molecules, we have non-polar molecules as well. As you have seen above we have classified SiF4 and BF3 as non-polar molecules. These are molecules that have no net dipole moment. Zero dipole moment can be a result of two conditions, one is that the atoms that are bonded together have no electronegativity difference e.g. hydrogen molecule. Second is that the net resultant dipole moment is zero as seen in the case of BF3.
