Question
Question: Which of the following is planar? (A) \[Xe{F_2}\] (B) \(Xe{O_3}F\) (C) \(Xe{O_2}{F_2}\) (D) ...
Which of the following is planar?
(A) XeF2
(B) XeO3F
(C) XeO2F2
(D) XeF4
Solution
Hybridization is the concept of mixing atomic orbitals into new hybrid orbitals (with different energies, shapes) suitable for pairing of electrons to form chemical bonds. Hybrid orbitals are the combination of standard atomic orbitals resulting in the formation of new atomic orbitals.
Complete step by step solution:
XeF4 Is planar as it has a square planar structure and sp3d2 hybridization.
Xenon tetrafluoride was the first discovered binary compound of a noble gas. It was produced by the chemical reaction of xenon with fluoride. The reaction is as shown:
Xe+2F2→XeF4
This is an exothermic reaction releasing an energy of 250KJmol−1.
Its molecular geometry is square planar. The bond angles are 90∘ or 180∘. The lone pairs lie on the opposite sides of the molecule basically at 180∘ from each other.
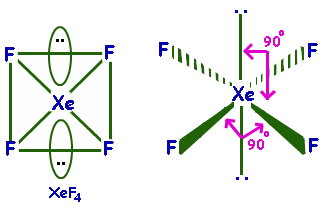
Hence, option D is correct
Note: XeF4 Molecule is nonpolar. It has octahedral geometry and square planar shape. The bonds are polar but the vector sum of the bond dipole is zero. The lone pair dipoles are equal in strength and oppose each other. Hence, it is a nonpolar molecule.
