Question
Question: Which of the following is not true of a detergent molecule? (a)- It has a nonpolar organic part an...
Which of the following is not true of a detergent molecule?
(a)- It has a nonpolar organic part and a polar group.
(b)- It is not easily biodegradable.
(c)- It is a sodium salt of fatty acid.
(d)- It is a surface active agent.
Solution
Detergent is a substance that is used to remove the dirt from other substances and it is similar in action to soaps. There is a minor difference in the soaps and detergent. One of the parts in the detergent is hydrophobic and one part of the detergent is hydrophilic.
Complete answer:
There are many types of detergents and soaps used to remove the dirt. So, let us understand what are detergent:
These are the products used in the washing process, which mix with oils, soil, and impurities to make them more water-soluble.
They're surface-active agents with a lot of strength. They're sulphonates of alkylbenzene.
They are manufactured artificially rather than being obtained from natural materials.
Since they are manufactured chemically, they are less biodegradable due to the inclusion of the sulfonate group, which is difficult for microorganisms in sewage to detach.
The consistency of detergent's washing operation is superior to that of soap.
Detergent has high reactivity, which is why it dissolves readily in both soft and hard water. There is no development of scum when they melt in rough water.
For long-chain carboxylic acids, they are ammonium or sulphonate salts.
Sodium lauryl sulphate (NaC12H25SO4 ), deoxycholic acid (C24H40O4 ), and other detergents are examples.
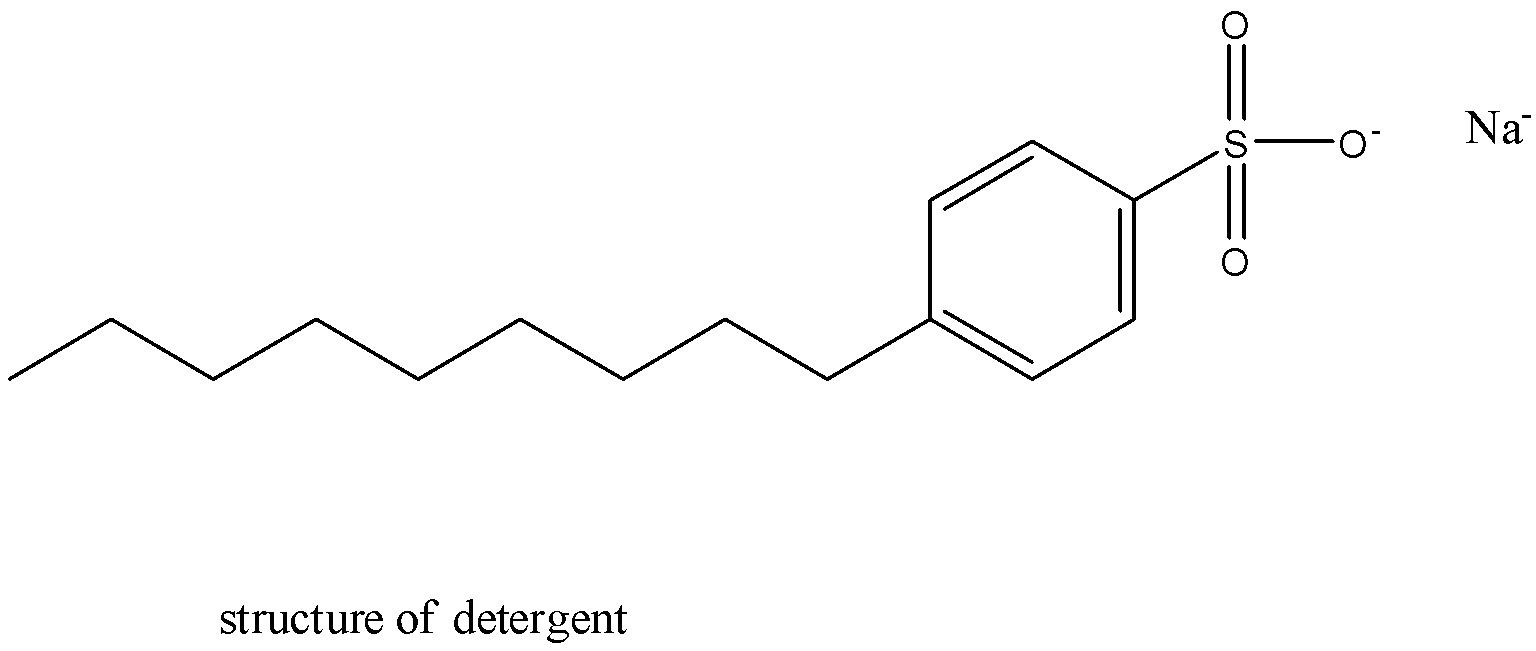
So, from the given options, sodium salt of fatty acids is wrong for detergent.
Therefore, the correct answer is an option (c).
Note:
Soaps should be used on our bodies because they are made from natural ingredients, but detergents cannot because of their high chemical content. They may cause skin irritation and may also be carcinogenic due to the high chemical content.
