Question
Question: Which of the following is not correct for zero order reaction? THIS QUESTION HAS MULTIPLE CORRECT ...
Which of the following is not correct for zero order reaction?
THIS QUESTION HAS MULTIPLE CORRECT OPTIONS
(A) 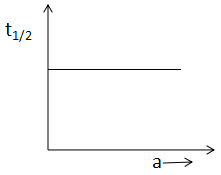
(B) 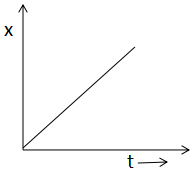
(C) 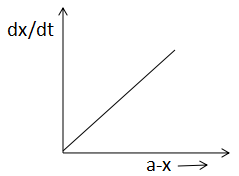
(D) 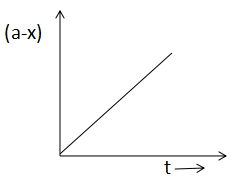
Solution
In A, the half life is constant throughout the reaction. In B, x is increasing with time. In graph C, rate and reactant is increasing in a straight line and in graph D, the half life is independent of the concentration.
Complete step by step answer:
In this question we have asked which of the following graphs is correct for zero order reaction. As we know, zero-order reaction is a reaction in which the rate does not vary with concentration of the reactants. So, the rate of reactions is always equal to the rate constant or we can say, the rate of these reactions is directly proportional to the 0th power of concentration of the reactant.
For graph A, the half-life period is independent of the concentration which occurs in first order reaction. Hence, graph D is for the first-order reaction.
Now look at graph B, which is showing that x is increasing with time which is correct. As we also know that [Ao]−[A]=kt . So, with an increase in time t, x increases and (a-x) decreases. So, graph B is also correct.
As we already mentioned that a-x is decreasing with increasing x and t or we can say a-x decreases when dtdx increases. Thus, Graph C is also correct.
Now, look at graph D. As we already mentioned that with an increase in time, x increases and a-x decreasing. So, option D is also correct
So, the correct options are B,C and D.
Note:
Example of a Zero-Order Reaction: The Haber process in which ammonia is formed from hydrogen and nitrogen gas. The reverse of this process in which the ammonia decompose to form nitrogen and hydrogen is a zero-order reaction.
