Question
Question: Which of the following is not aromatic? A.Benzene B.cyclooctatetraenyl dianion C.tropylium ion...
Which of the following is not aromatic?
A.Benzene
B.cyclooctatetraenyl dianion
C.tropylium ion
D.cyclopentadienyl cation
Solution
For a molecule to be aromatic, it should be cyclic, planar, must fulfil Huckel’s rule and must be conjugated. His rule states that if a cyclic, planar molecule has 4n+2 π electrons, it is considered To be aromatic.
Complete step by step answer:
Non-aromatic does not follow Huckel’s rule. are highly unstable and highly reactive.
A.Benzene is cyclic, planar and has 6 π electrons thus fulfilling Huckel’s rule. It is therefore aromatic.
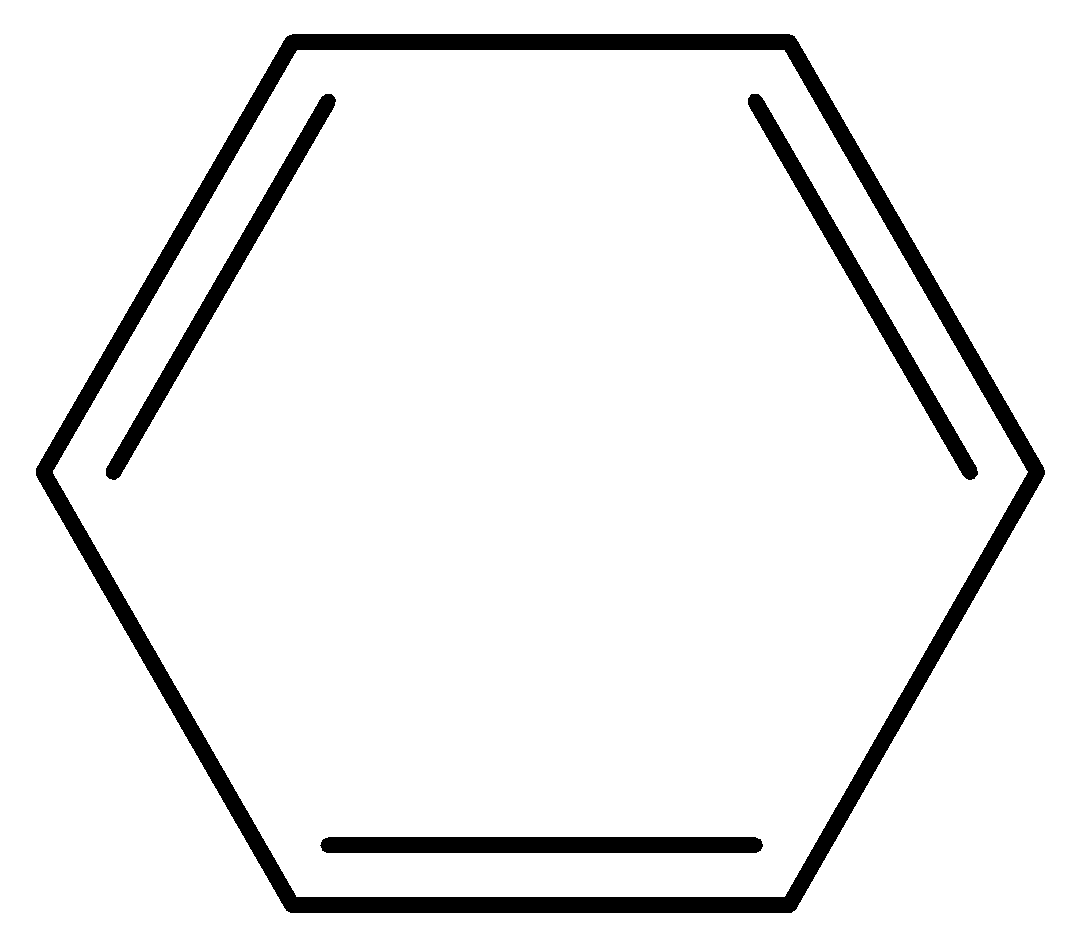
6 π electrons
B.Cyclooctatetraenyl Dianion has 10 π electrons thus fulfilling Huckel’s rule. It is cyclic as well as planar. It is therefore aromatic.
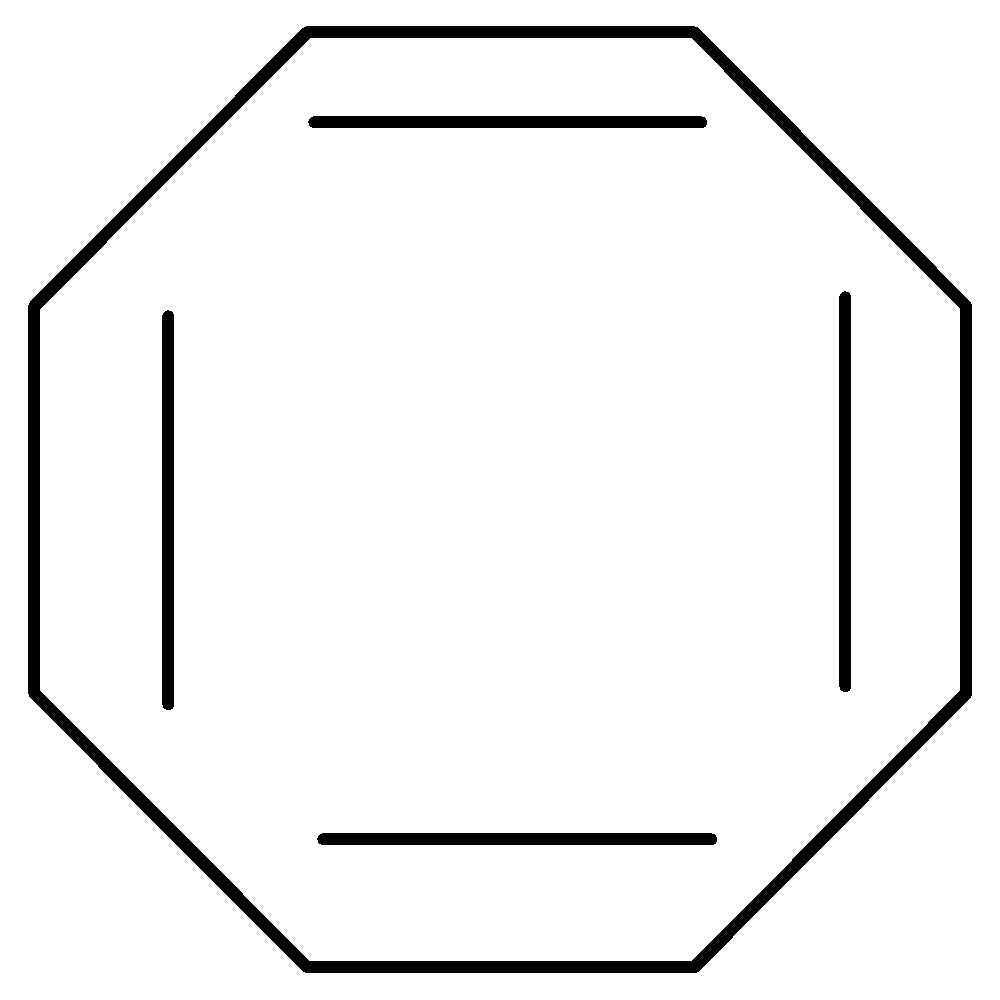
10 π electrons
C.Tropylium Ion has 6 π electrons. Thus it also fulfils Huckel’s rule. It is cyclic as well as planar. Therefore, it is also aromatic.
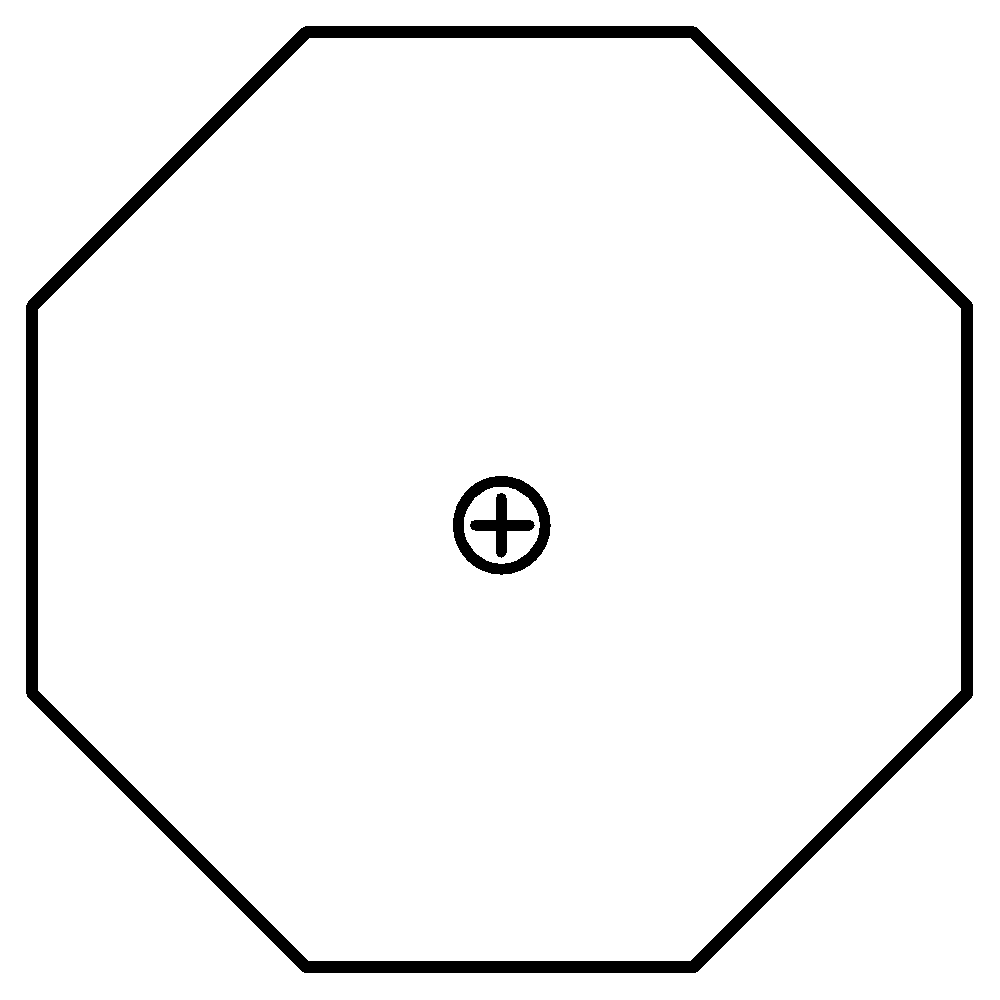
6 π electrons
D.In Cyclopentadienyl cation, if a hydride anion (H−) is removed from the
sp3 hybridized ring carbon, the cyclopentadienyl cation is formed, and hybridization of sp3 ring carbon is altered to sp2 .
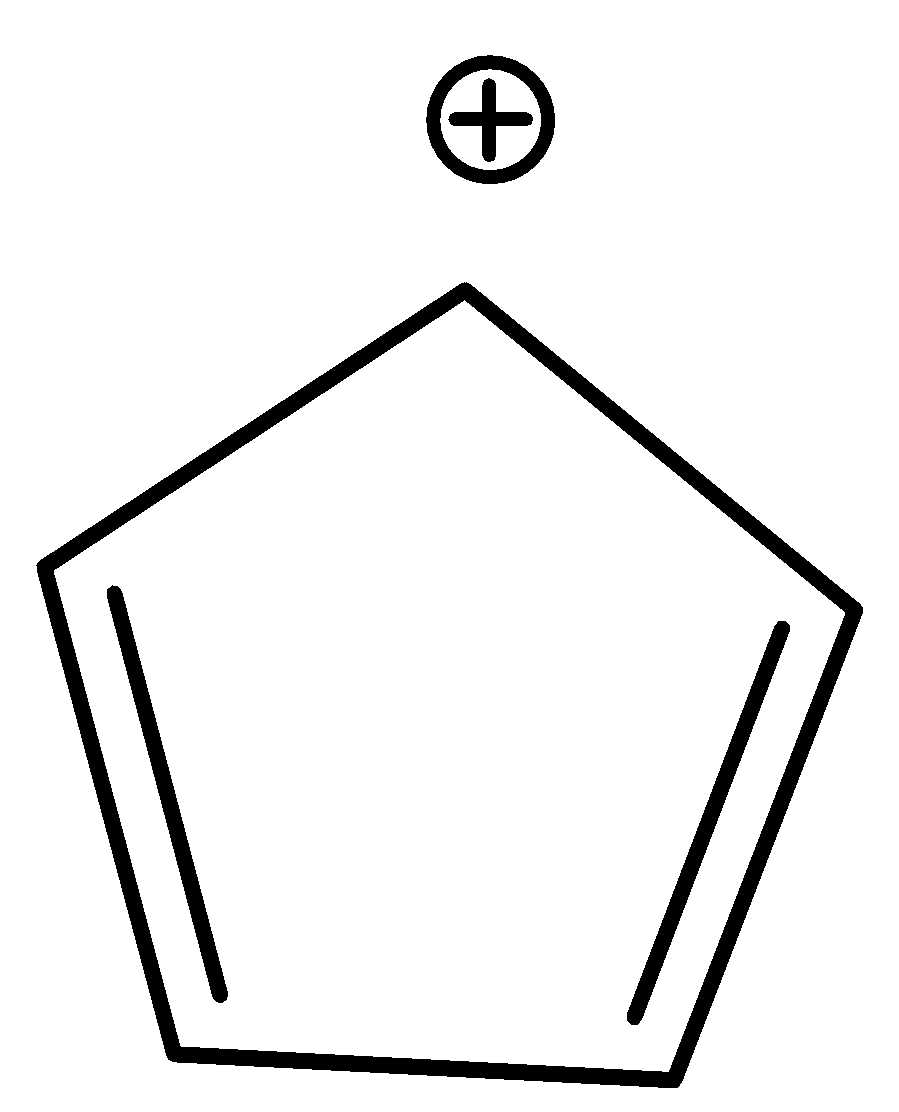
4 π electrons
Thus, the cyclic cyclopentadienyl cation is planar and possesses a cyclic uninterrupted π electron cloud. However, it does not follow Huckel’s rule as it has 4 π electrons in a conjugated system. Thus, it is antiaromatic.
Hence option D is correct.
Note:
Molecules may change shape, becoming non-planar to avoid the instability of anti-aromaticity and therefore breaking some of the π interactions.
