Question
Question: Which of the following is not an example of addition polymer? A.Polythene B.Polystyrene C.Neop...
Which of the following is not an example of addition polymer?
A.Polythene
B.Polystyrene
C.Neoprene
D.Nylon 6,6
Solution
Polymers are large molecules having high molecular weight formed by a reaction called polymerisation. The repeating units in a polymer are derived from small molecules called monomers.
Complete step by step answer:
Small molecules called monomers combine each other under certain conditions to form molecules of large molecular size. This reaction is called a polymerisation reaction. Two types of polymerisation reactions are there- addition polymerisation and condensation polymerisation.
In addition to polymerisation, the monomer units simply combine with each other. It does not produce any co-products. But in condensation polymerisation, monomers combine each other by eliminating small molecules like water or methanol.
Now let us discuss each of the given options.
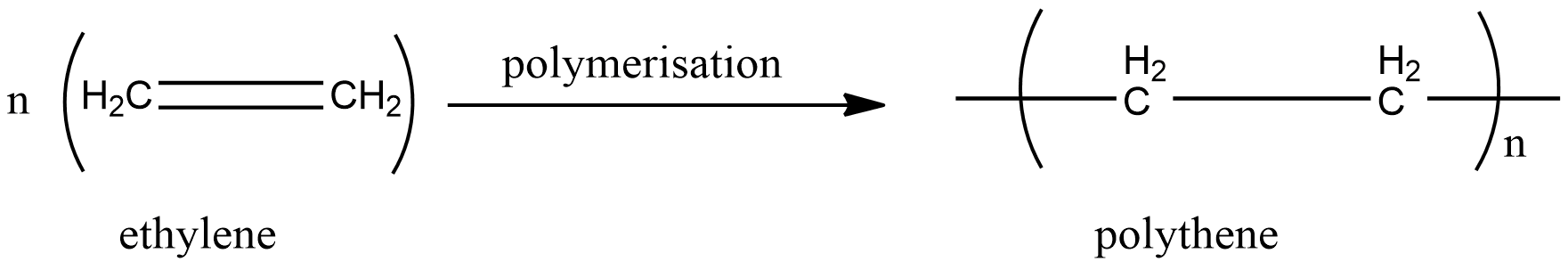
a.Polythene
As the name suggests, polythene is formed by the polymerisation is ethylene. The double bond in ethylene breaks and each ethylene molecule combines each other to form polythene. There are no side products. Hence polythene is an addition polymer.
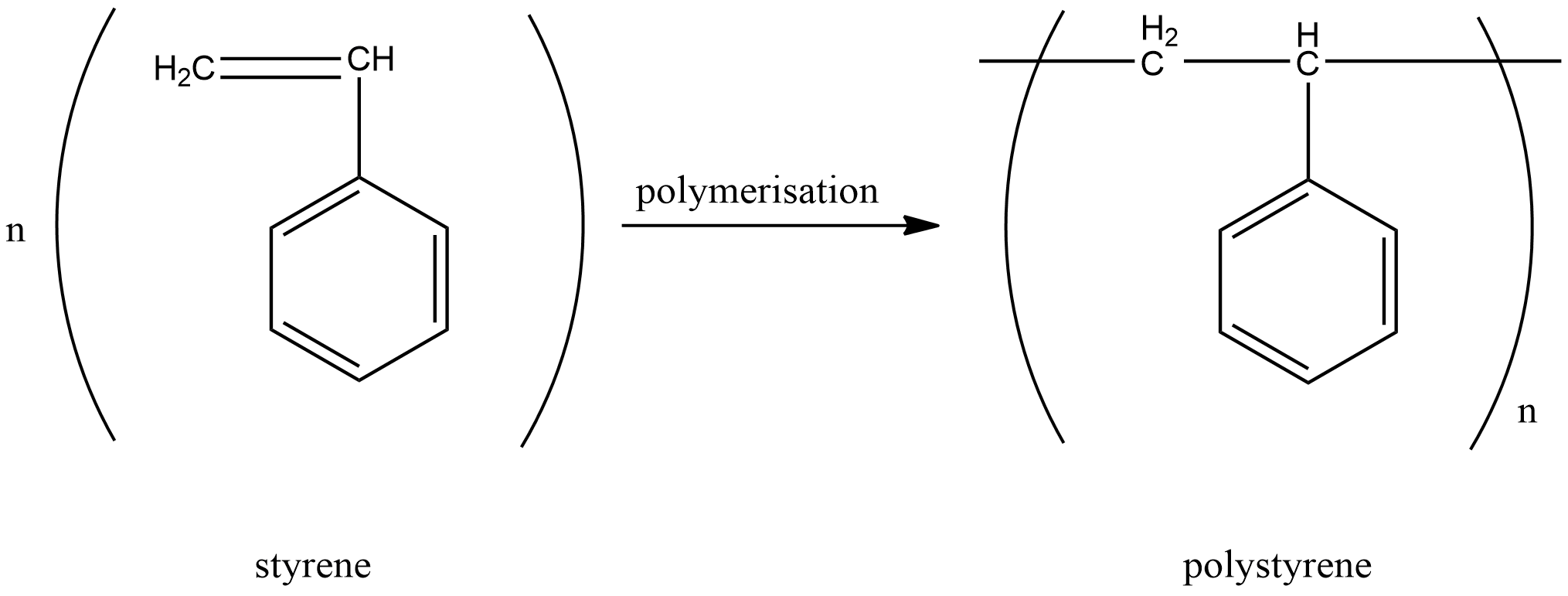
B.Polystyrene
Monomer units in polystyrene are styrene. Similar to polythene, polystyrene is also formed by breaking the double bond and linking the monomeric units together. Hence this is also an addition polymer.
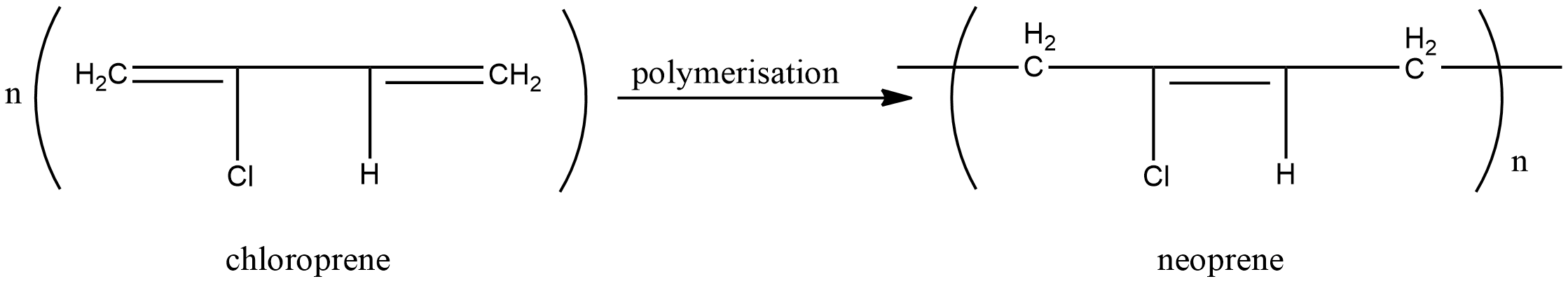
C.Neoprene
Monomeric unit neoprene is chloroprene. Chloroprene molecules link together without elimination of any other products to form neoprene. Hence neoprene is also an addition polymer.
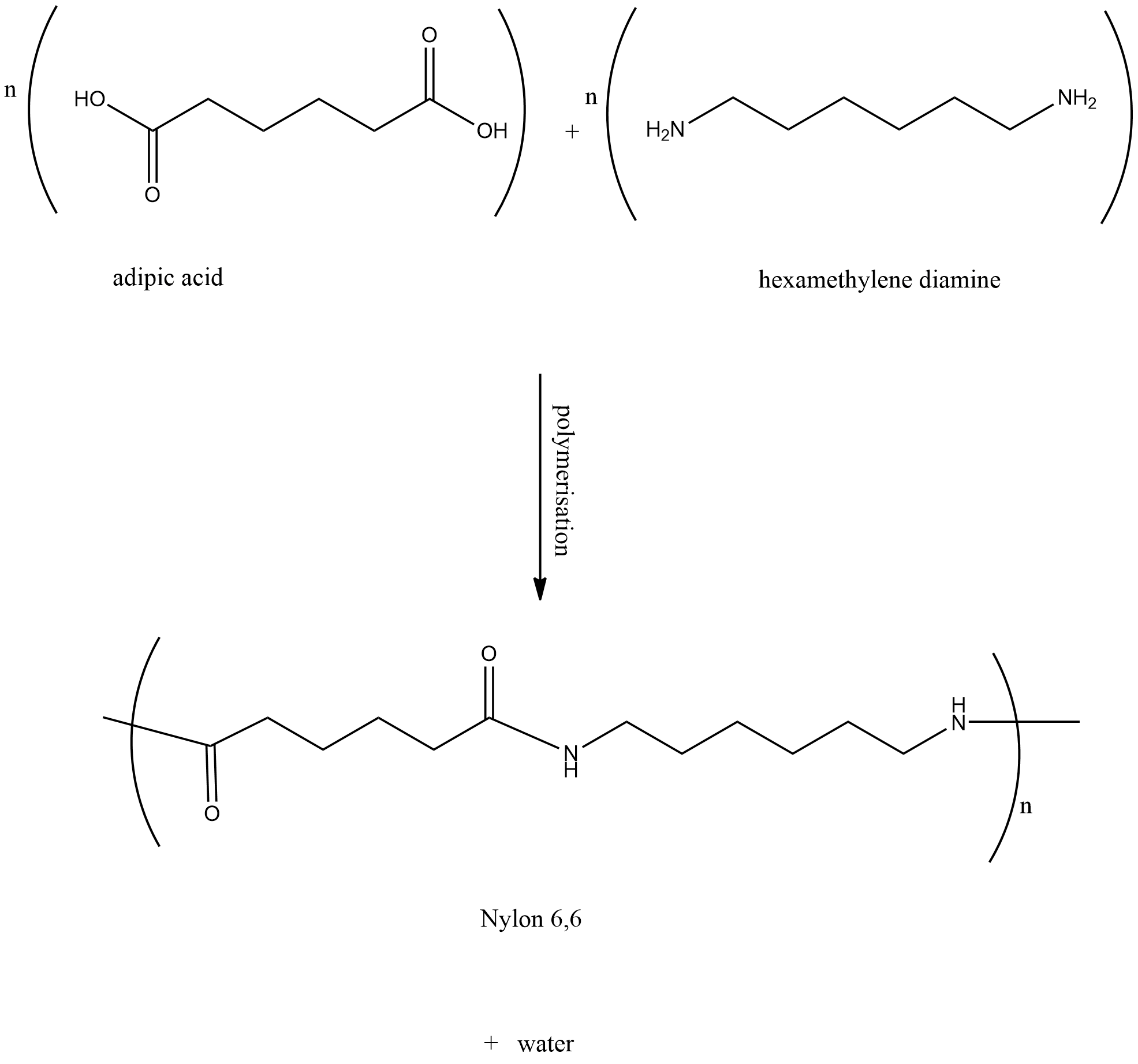
D.Nylon 6,6
Nylon 6,6 consist of hexamethylene diamine and adipic acid as monomeric units. This reaction results in the elimination of water molecules. Hence we can say that Nylon 6,6 is a condensation polymer of hexamethylene diamine and adipic acid.
Option D is correct.
Note:
The molecules having one or two functional groups usually undergo condensation polymerisation. The monomer units can be the same or different. Nylon 6,6 is a synthetic condensation polymer. There are also naturally occurring condensation polymers. e.g. polypeptide chains of protein, cellulose etc.
