Question
Question: Which of the following is not an aromatic compound? A)  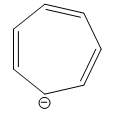
B) 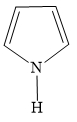
C) 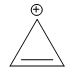
D) 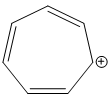
Solution
Follow the Huckel rule of aromaticity. A conjugated, cyclic planar compound having (4n+2) π electrons is aromatic.
Complete answer:
If the number of pi electrons in the compound is not equal to (4n+2) then the compound is non aromatic. For n=0,1,2,3... the value of (4n+2) is 4(0)+2=2,4(1)+2=6, 4(2)+2=10, 4(3)+2=14... respectively.
Hence, if the number of pi electrons in a compound is not equal to 2,6,10, 14… then it is not an aromatic compound. In the option A ) cycloheptatrienyl anion has 8 pi electrons. Thus the number of pi electrons in cycloheptatrienyl anion is not equal to 2,6,10, 14…
Hence, cycloheptatrienyl anion is not an aromatic compound.
Hence, the correct option is option A ).
Additional Information: The compounds/ions in options B ) and D ) have 6 pi electrons. They are cyclic, planar and conjugated systems of pi electrons. Hence, compounds/ions of options B ) and D ) are aromatic.
The ion of option C ) has 2 pi electrons. It is conjugated, cyclic and planar. Hence, it is aromatic in nature.
Note: While counting the number of pi electrons, each double bond corresponds to a pair of electrons. Each lone pair of electrons corresponds to a pair of electrons. The orbital in which the lone pair of electrons is present, should be parallel to the p orbitals containing pi electrons. If a molecule/ion contains (4n+2) π pi electrons, but it is non cyclic, non planar or non conjugated, then it is not an aromatic compound.
