Question
Question: Which of the following is not an aliphatic compound? A.Ethylene oxide B.Isobutylene C.Neopenta...
Which of the following is not an aliphatic compound?
A.Ethylene oxide
B.Isobutylene
C.Neopentane
D.Acetaldehyde
Solution
An aliphatic compound can be understood as a straight chain is non – aromatic in nature. To solve this question, we must draw the molecular structures of the compounds to identify the one which is not an aliphatic compound
Complete Step-by-Step Answer:
Before we move forward with the solution of the given question, let us first understand some important basic concepts.
The molecular structures of the given compounds can be drawn on the basis of breaking the compound’s name using the IUPAC rules. Hence, the molecular structures of the given compounds can be given as follows:
1.Ethylene oxide: Ethylene oxide can be primarily understood as a compound with 2 carbon atoms. Also included in the molecule is an oxygen atom. Since the oxidation state of oxygen is (-2). Hence, it cannot be singly bonded with any carbon atom. And we cannot doubly bond the oxygen with the carbon with the carbon atom because it would then form a carbonyl compound like aldehyde or ketone, which is not mentioned in the name of the compound. Hence the structure of ethylene oxide is a ring-based structure with 2 carbon atoms and 1 oxygen atom. The structure can be given as:
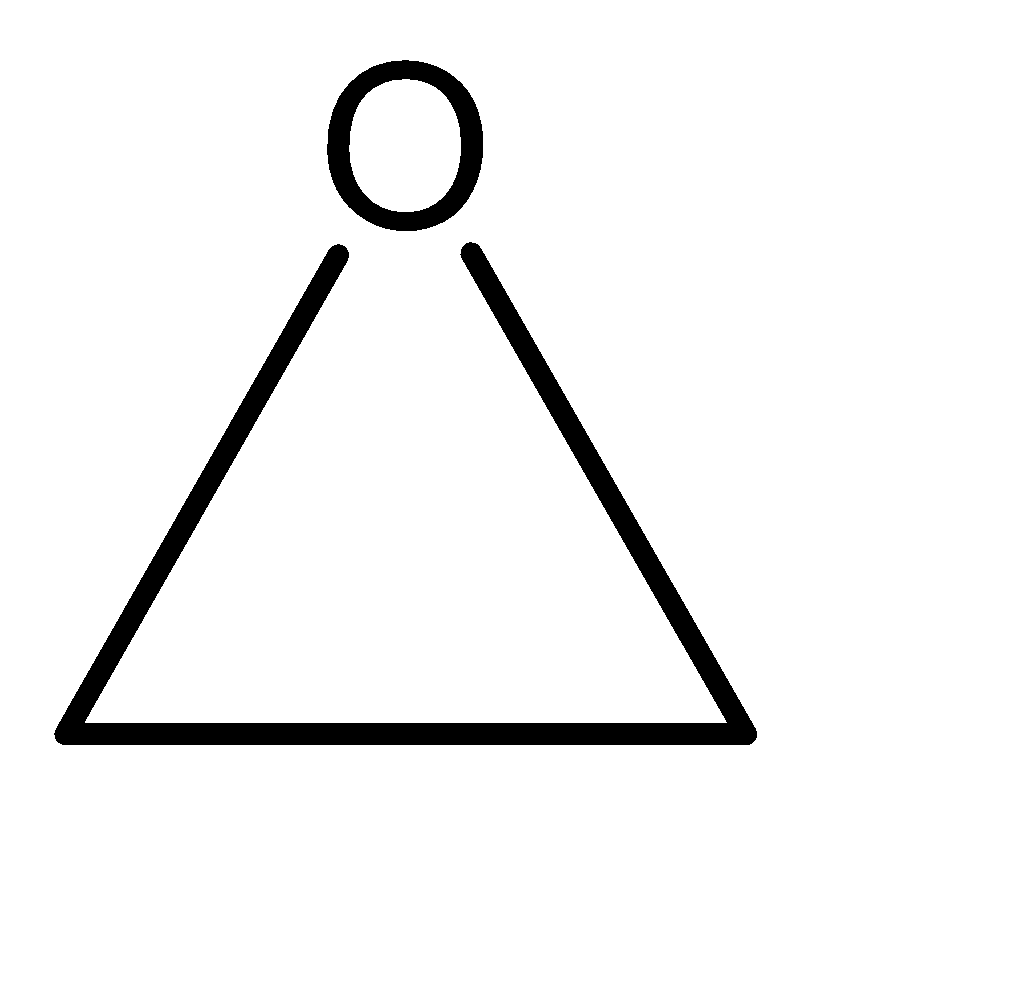
2.Isobutylene: Isobutylene can be primarily understood as a compound with 4 carbon atoms. Iso – compounds have single carbon chains at the position 02 carbon of the parent chain. Also, the structure has a pi bond. Hence the molecular structure of isobutylene can be given as:
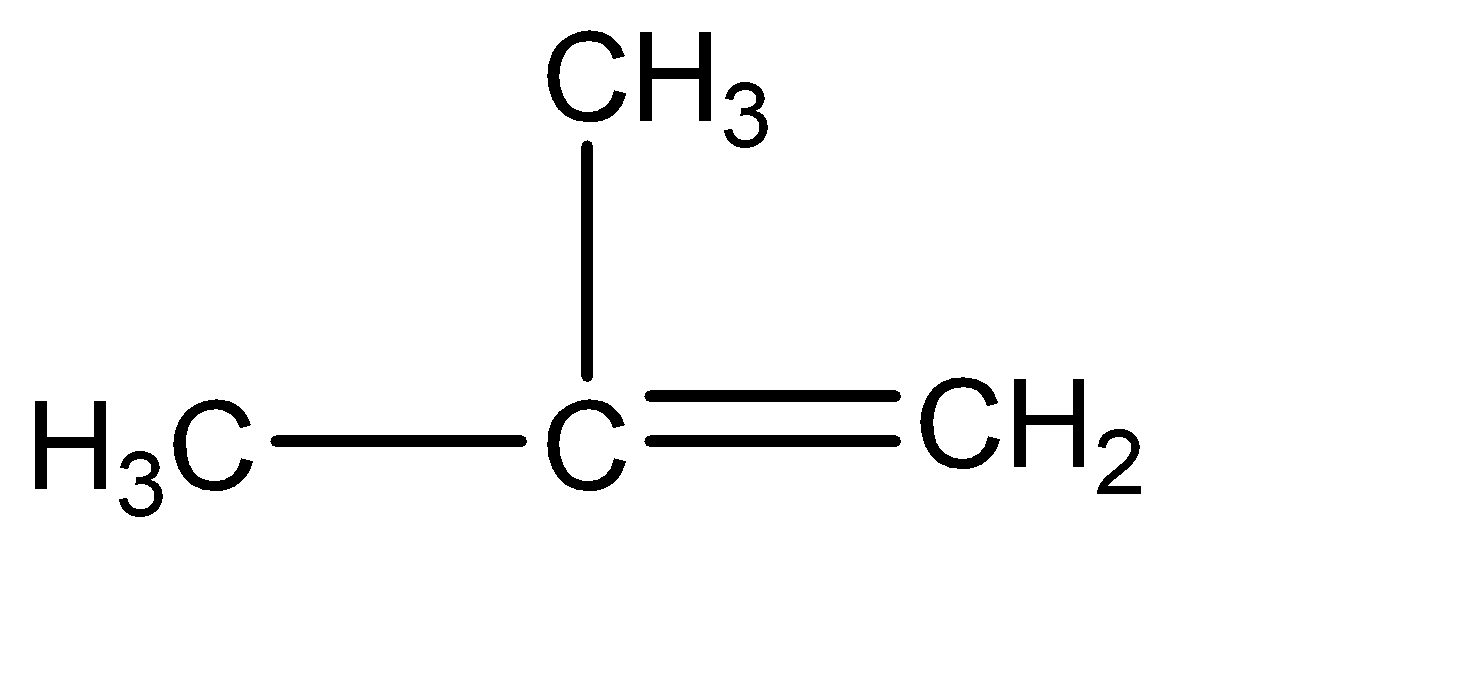
3.Neopentane: Isobutylene can be primarily understood as a compound with 5 carbon atoms.
Neo – compounds have two carbon chains at the position 02 carbon of the parent chain. Hence the molecular structure of neopentane can be given as:
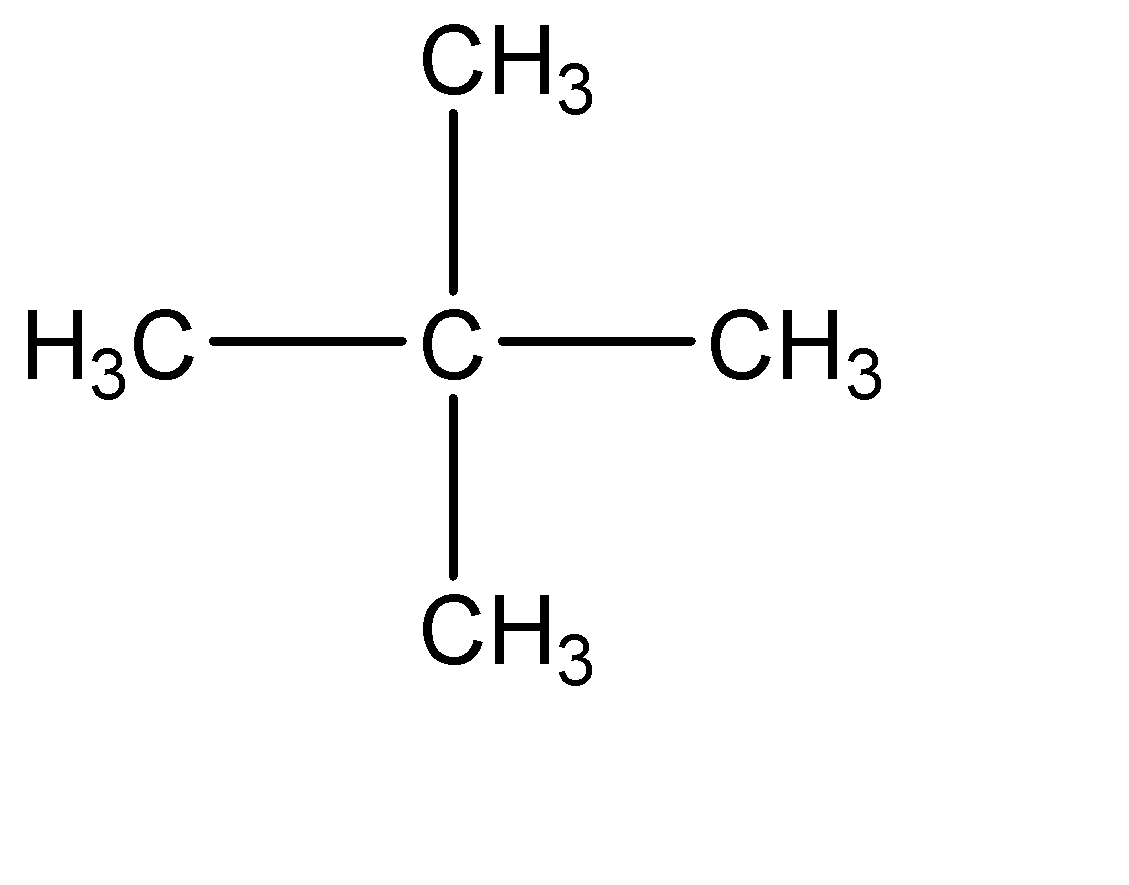
Acetaldehyde: Acetaldehyde can be primarily understood as the compound with two carbon atoms out of which one carbon atom has a carbonyl group. hence the molecular structure of acetaldehyde is:
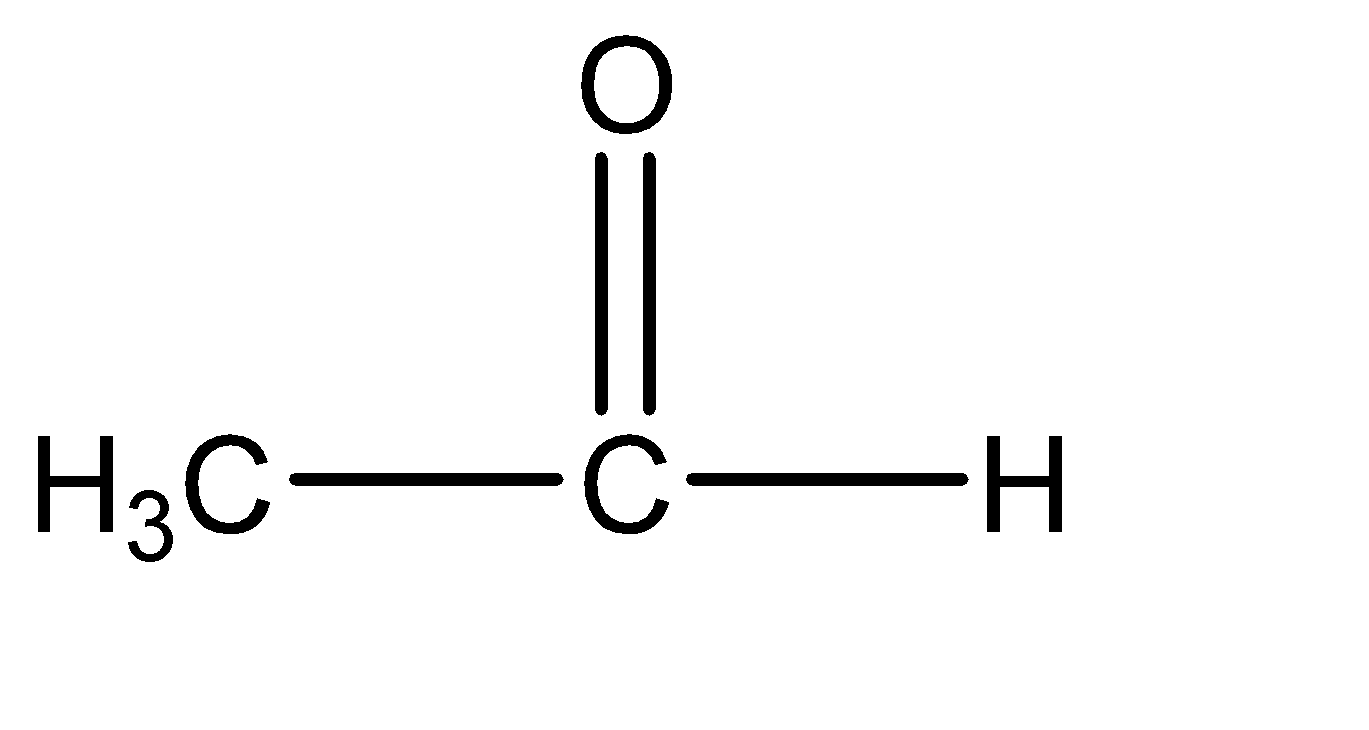
Hence, Option A is the correct option
Note: Aliphatic compounds can be cyclic in nature. The only condition is that the cyclic structure should not be conjugated in such a way that it obeys Huckel's Rule. Obeying Huckel's Rule would make the compound aromatic. An example of a cyclic aliphatic compound is cyclohexane.
