Question
Question: Which of the following is not an acidic salt? A.\({\text{Na}}{{\text{H}}_{\text{2}}}{\text{P}}{...
Which of the following is not an acidic salt?
A.NaH2PO2
B.NaH2PO3
C.NaH2PO4
D.All of these
Solution
Any salt that has replaceable hydrogen atoms is known as an acidic salt. The aqueous solutions of acidic salts turn blue litmus red. Thus, the aqueous solutions of acidic salts are acidic in nature.
Step by step answer:
The salt NaH2PO2 is formed by the neutralization of weak acid H3PO2 and strong base NaOH.
The structure of H3PO2 is as follows:
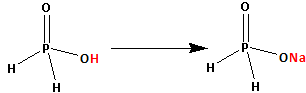
Thus, H3PO2 has only one replaceable hydrogen atom. This one hydrogen atom is replaced by one sodium atom and NaH2PO2 is formed.
Thus, NaH2PO2 is not an acidic salt.
Thus, option (A) is correct.
The salt NaH2PO3 is formed by the neutralization of weak acid H3PO3 and strong base NaOH.
The structure of H3PO3 is as follows:
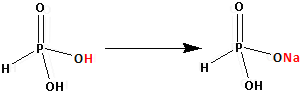
Thus, H3PO3 has two replaceable hydrogen atom. One hydrogen atom is replaced by one sodium atom and NaH2PO3 is formed. One replaceable hydrogen atom is still remaining.
Thus, NaH2PO3 is an acidic salt.
Thus, option (B) is not correct.
The salt NaH2PO4 is formed by the neutralization of weak acid H3PO4 and strong base NaOH.
The structure of H3PO4 is as follows:
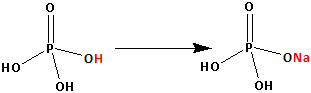
Thus, H3PO4 has three replaceable hydrogen atom. One hydrogen atom is replaced by one sodium atom and NaH2PO4 is formed. Two replaceable hydrogen atoms are still remaining.
Thus, NaH2PO4 is an acidic salt.
Thus, option (C) is not correct.
Thus, NaH2PO2 is not an acidic salt.
Thus, the correct option is (A). NaH2PO2.
Note: In the acids, the hydrogen atoms that are attached to the oxygen atom are known as replaceable hydrogen atoms. Hydrogen atoms attached to phosphorus directly are not replaceable.
