Question
Question: Which of the following is not a use of baking soda? A.In medicines as antacids B.As a component ...
Which of the following is not a use of baking soda?
A.In medicines as antacids
B.As a component of baking powder
C.In removing permanent hardness of water
D.In fire extinguishers
Solution
-Antacids are used for medical purposes to neutralise the acid which is present in the stomach, as increase in concentration of acid in the stomach can cause peptic ulcers or other serious medical conditions.
-Baking soda has a number of applications for the household purposes, as the name suggests it can be used for baking because of the leaving nature of carbon dioxide from the bicarbonate group.
Complete step by step answer:
Before we jump to the application of baking soda, we must first know the chemical formula and name of the baking soda. The chemical formula of baking soda is NaHCO3, and the chemical name of the baking soda is sodium bicarbonate. It contains a sodium atom which is attached to one of the oxygen atoms out of the three oxygens which are attached to the carbon. The hydrogen is attached to one of the oxygen forming a hydroxide group. Since it is difficult to visualize the structure just with the help of the formula, we will be considering the structure of sodium bicarbonate for a better understanding.
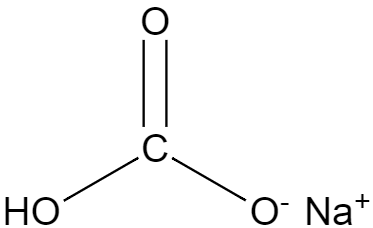
Now, we can see that one of the oxygen has a double bond with carbon, hence satisfying the valency of the oxygen, and another hydroxide group so attached to the same carbon satisfying its valency, and finally the third oxygen is sharing one bond with the carbon, satisfying the valency of carbon and another ionic bond is present between the anionic oxygen and the cationic sodium atom.
Now if we consider the first option it questions the use of baking soda as an antacid. Antacids are the substances which are consumed in order to neutralize the acid which is present in our stomach. Since sodium bicarbonate can act as a base, it can be used to neutralize the acid which is present in the stomach. Hence the first option is not correct, as baking soda is in fact used as an antacid.
We know that sodium bicarbonate is very commonly used to make baking powder, in other words baking soda is one of the main constituents of baking powder. Hence the next option is also incorrect.
Baking soda cannot be used in removing permanent hardness of water as it does not have the capacity to form soluble compounds with the calcium and magnesium ions present in the hard water. So the option C is correct.
Baking soda can be used to make fire extinguishers because it is not inflammable, hence the option D is incorrect.
So the appropriate option would be option C.
Note: Baking soda has a number of applications, but most common is it can be used in the process of baking, because of its properties to leave the carbon dioxide which causes the dough to rise, and hence the volume of the dough increases.
-One of the main constituents of baking powder is baking soda, but it also contains some acid which makes it different from the baking soda.
