Question
Question: Which of the following is NOT a property of red phosphorus? (A) Insoluble in carbon disulphide (...
Which of the following is NOT a property of red phosphorus?
(A) Insoluble in carbon disulphide
(B) It does not show chemiluminescence by action of air
(C) It forms phosphine when treated with hot sodium hydroxide solution
(D) It is non-poisonous
Solution
Red phosphorus is an allotrope of the element phosphorus. It is also a derivative of P4 molecule. As it is deep red in colour it is called red phosphorus. It has a powdery texture. It exists in amorphous form in nature. It has a melting point of 860K. It is odourless and is solid at standard temperature and pressure.
Complete step by step answer:
The structure of red phosphorus resembles that of P4 molecule. The structure of red phosphorus is given below:
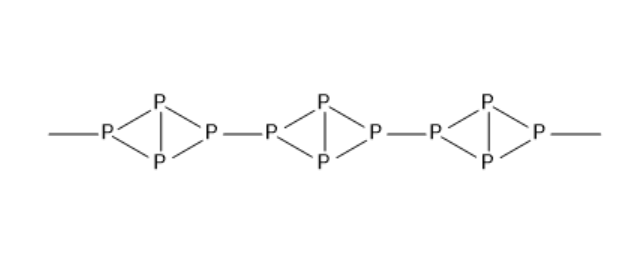
Red phosphorus is not soluble in water, alkalies and carbon disulphide because it exists in a polymeric structure. Under normal conditions, it remains steady. So, it does not show chemiluminescence. When white phosphorus is in contact with sodium hydroxide and water, it gives phosphine and also sodium hypophosphite. But red phosphorus does not give phosphine. Red phosphorus is considered non-toxic.
Therefore, option C is correct.
Note: Notice the word not in question carefully. Many students think that the question is property of red phosphorus and they choose option A. The structure of compounds helps in determining properties. So, notice that red phosphorus has a polymeric structure in which four phosphorus atoms together form a tetrahedral structure. Red phosphorus is the intermediate state between white phosphorus and violet phosphorus.
