Question
Question: Which of the following is not a peroxy acid? A) Perphosphoric acid B) Pernitric acid C) Perdi ...
Which of the following is not a peroxy acid?
A) Perphosphoric acid
B) Pernitric acid
C) Perdi Sulphuric acid
D) Perchloric acid
Solution
Peroxy acids, also known as peracids, are generally strong oxidants and have an −OOH group. When the OH group of the oxy group is replaced by an acidic −OOH group, a peroxide acid is formed. This group exchange occurs because, in the group, oxygen and hydrogen are covalently bonded and easily decomposed into cations and hydride anions. The new bond now formed with an alkyl or benzyl group is called a peroxy bond.
Complete answer:
Perphosphoric acid, Pernitric acid, and Perdi Sulphuric acid are examples of peroxy acids as they contain −OOH . Perchloric acid is not an example of peroxy acid. The chemical formula of HClO4 .
And the structure is –
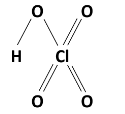
As this does not contain −OOH , so the correct option D) Perchloric acid.
Note:
As peroxy acids are very strong oxidation agents. Therefore, they readily add oxygen to alkenes to convert them into their corresponding epoxides. Similarly, they convert ketones to esters and amines compounds to nitro compounds, nitroso compounds, or amino oxides.
