Question
Question: Which of the following is not a Lewis acid? (a)- \(FeC{{l}_{3}}\) (b)- \(BC{{l}_{3}}\) (c)- ...
Which of the following is not a Lewis acid?
(a)- FeCl3
(b)- BCl3
(c)- ZnCl2
(d)- NH3
Solution
Lewis explained that an acid accepts a pair of electrons and the base donates a pair of electrons. So, we can identify the compound which has electrons to donate is not a Lewis acid.
Complete step by step answer:
According to Lewis, an acid can be defined as a substance that can accept a pair of the electron, and a base is that substance that can donate a pair of electrons.
So, the acid must be an electron-deficient compound and the base must be an electron-rich compound.
In FeCl3, the iron atom has an incomplete octet. The iron atom is bonded with three chlorine atoms with electrons and it can accept a pair of the electron, therefore it is a Lewis acid.
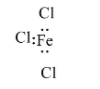
In BCl3, the boron atom has an incomplete octet. The boron atom is bonded with three chlorine atoms with electrons and it can accept a pair of the electron, therefore it is a Lewis acid.
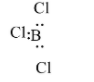
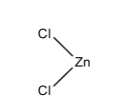
In ZnCl2, the zinc atom has an incomplete octet. The zinc atom is bonded with two chlorine atoms, this means that zinc is in +2 oxidation state, hence it can accept a pair of electrons. Therefore, it is a Lewis acid.
In NH3, the nitrogen atom is bonded with three hydrogen atoms, but the valence electrons in nitrogen are 5. So it has a pair of electrons that are non-bonded. So, it donates a pair of electrons. It is a Lewis base.
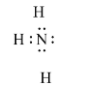
So, the correct answer is an option (d)- NH3
Note: Some other examples of Lewis acid in molecular form are BF3. Cations that acts as Lewis acid are Ag+,Cu2+,Fe3+, etc. The molecules having empty d-orbital also act as Lewis acid, SiF4.
