Question
Question: Which of the following is most stable: A.
B.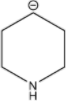
C.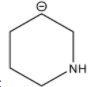
D.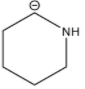
Solution
Stability of the carbanion depends upon the delocalization of the negative charge. The higher the delocalization of the negative charge, the higher will be the stability of the carbanion. The stability of the carbanion is also dependent upon the presence of an electron withdrawing group, higher the number of electron withdrawing groups higher will be the stability of the carbanion and vice-versa. For example the stability order of carbanion is as follows.
Complete step by step answer:
Among the given structures of the carbanion. The presence of the amine group stabilizes the negative charge. In this case, nitrogen is an electronegative element that shows an electron-withdrawing effect. Due to the electron-withdrawing nature of the nitrogen, the carbanion gets stability.
Now inductive effect depends upon the distance. With increasing the distance between the amine group and negative charge, the stability of the carbanion decreases and vice-versa.
Therefore, based on the inductive effect the stability is highest for option D. As the amine group is in the proximity which stabilizes the carbanion.
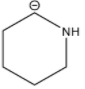
So, the correct option is D.
Note:
A carbocation is a molecule in which a carbon atom has a positive charge and three bonds. It is defined as any even-electron cation that possesses a significant positive charge on the carbon atom and the alpha carbon refers to the first carbon atom that attaches to a functional group, such as a carbonyl.
