Question
Question: Which of the following is most basic: (A) 
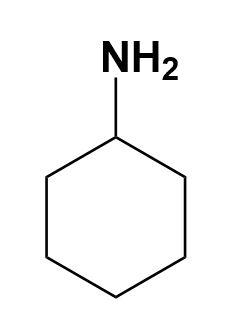
(B)
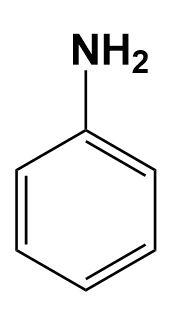
(C)
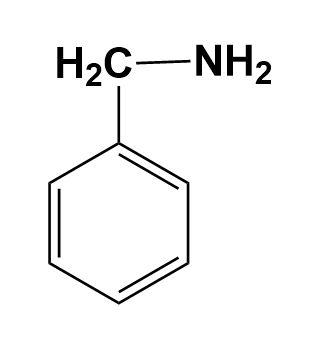
(D)
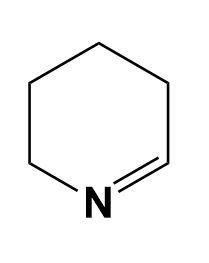
Solution
Basically, the basicity of a molecule is determined and compared by the presence of an electron donating group and here, availability of a lone pair of electrons on nitrogen atoms.
Here, in this illustration we need to find the relative basicity of the given compounds and decide the more basic compound.
Complete answer:
Let us discuss the phenomenon of basicity and focus on the basicity of nitrogen containing compounds;
The two phenomena of basic chemistry are acidity and basicity which is the measure of extent of reaction and are based on the same chemical reaction taking place in nature.
Basicity is generally the availability and extent of stability of electrons in an atom or a molecule. The availability of electron pairs on the molecule gives the allowance of its donation which is the basic definition of basicity.
There are trends of basicity for the modern periodic table;
The basicity will be higher when we have less electronegative element as the lone pair on it will be less stable.
The basicity decreases as we go down the group as down the group electron density is eventually reduced.
In the given illustration, the cyclohexylamine is more basic than other three compounds i.e. aniline, benzylamine and 1-piperideine as the electron pair is easily available on the nitrogen of cyclohexylamine compared to other compounds.
Therefore, cyclohexylamine is more basic i.e. option (A) is correct.
Note:
There are some trends for the basicity in nitrogen containing compounds i.e. amines, aniline, amides, etc., as well. Some of them are:
Basicity increases with increase in negative charge on a nitrogen atom.
Basicity decreases when the compounds are stabilised by resonance and conjugations.
Basicity decreases with the inductive effects.
Basicity of a nitrogen is decreased when it is attached to a pi-acceptor.
Basicity of a nitrogen is increased when it is attached to a pi-donor.
