Question
Question: Which of the following is most acidic? 
Solution
To determine the acidic compound first we should know what is acidic compounds and how to check the acidic strength of the compound. The compound which donates protons is known as acid. If after the donation of a proton the formed anion is stabilized by any factor then the compound will be acidic. If not the compound will be not acidic or we can say less acidic.
Complete answer: We will remove one proton from each of the given compounds and will form their anion then we will check the stabilizing factor for the anion. More stable the formed anion will cause easy loss of proton so, more will be acidity.
The anion of each compound is shown as follows:
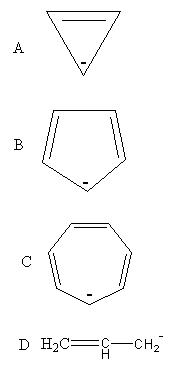
In a cyclic compound, we check the stability of the anion by the Huckel rule. According to which the compound having 4nπ delocalised electrons are anti-aromatic and the compound having 4nπ+2 delocalised electrons are aromatic. The aromatic compounds are more stable than the anti-aromatic.
In an anion of compound A, the 4π electrons are delocalising. When we put n = 1, in 4nπ formula we get 4π. So, anion of compound A is anti-aromatic so, the compound A will not lose proton easily due to anti-aromatic character so, A is not acidic.
In an anion of compound B, 6πelectrons are delocalising. When we put n = 0, in 4nπ+2 formula we get . So, anion of compound B is aromatic and hence stable, so it will lose proton and it is acidic.
In an anion of compound C, the 8π electrons are delocalized. When we put n = 2, in 4nπ formula we get8π. So, anion of compound C is anti-aromatic so the compound C will not lose proton easily due to anti-aromatic character, so C is not acidic.
The stability of the ion of the non-cyclic compound is determined based on delocalization of charge, hyper-conjugation, or inductive effect. In the anion of compound D, the negative charge can delocalize at all three carbon as follows:
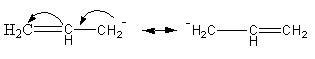
So, compound D is also acidic.
Now, in compound B, the negative charge is stabilised by delocalization on five carbons where in compound D, negative charge is stabilised by delocalization on three carbons only. More the delocalization more will be the stability so, the anion of compound B is more stable than anion of compound D. So, compound B will lose protons more easily than compound D. So, compound B is most acidic.
Therefore, the correct answer is (D).
Note: The acids form anion so, we always check the stability of anion to determine the acidity. The electron-withdrawing (-M) resonance and (-I) inductive effect stabilises the anion so, increases the acidity. The electron-donating (+M) resonance and (+I) inductive effect destabilises the anion so, decreases the acidity. The compound forming aromatic anions is more acidic than the compound forming anti-aromatic anion.
