Question
Question: Which of the following is more reactive in a hydrolysis reaction to make butanoic acid? A) Butanoi...
Which of the following is more reactive in a hydrolysis reaction to make butanoic acid?
A) Butanoic anhydride
B) Methyl butanoate
C) N-methylbutanamide
D) Butanenitrile
Solution
The greater is the unsaturation the easier will be to hydrolyse a given compound. The most unsaturated compound in the above given option will be most reactive in the formation of butanoic acid.
Complete step by step solution:
Hydrolysis refers to the process of breaking a molecule in the presence of water.
Acid anhydride, amide and esters all hydrolyse to form carboxylic acid. The nitrile functional group is most reactive because it contains a triple bond in between carbon and nitrogen. The process of hydrolysis is very efficient. The nitrile functional group is −C≡N.
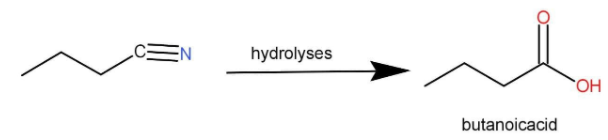
Hence, the correct option is D.
Additional information:
Butyric acid is another name for butanoic acid. It is named so because it is found in butter. It is oily and colourless liquid. However it is not found abundantly but its ester do. It is soluble in water and ethanol. It is used in the preparation of CAB that is cellulose acetate butyrate. CAB is used in the preparation of various tool parts, coating and any more. It is less degradable than cellulose acetate. It has a very strong odour. For this reason it is used as a fishing bait additive. It is commercially used in crab baits.
Note:
Butyric acid is one of the short chain fatty acids. It is found in animal fat, plant oils, bovine milk, breast milk, butter, parmesan cheese. It is produced by anaerobic fermentation. Animals have a very strong detection ability of butyric acid. Dogs can even smell this acid at 10 ppm concentration.
