Question
Question: Which of the following is least stable:- (A) \(C{H_3} - \mathop {CH}\limits^ + - C{H_3}\) (B) \(...
Which of the following is least stable:-
(A) CH3−CH+−CH3
(B) CH3−CH2−CH2+
(C)
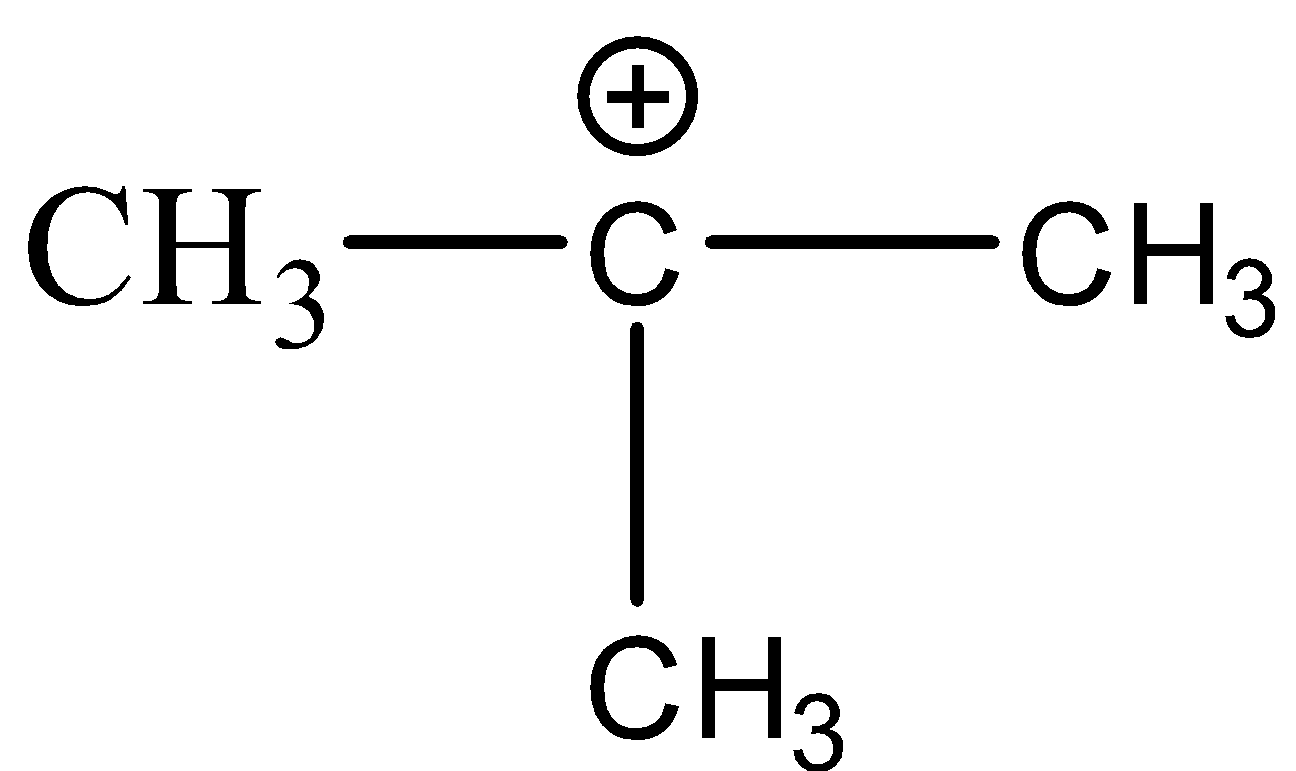
(D)
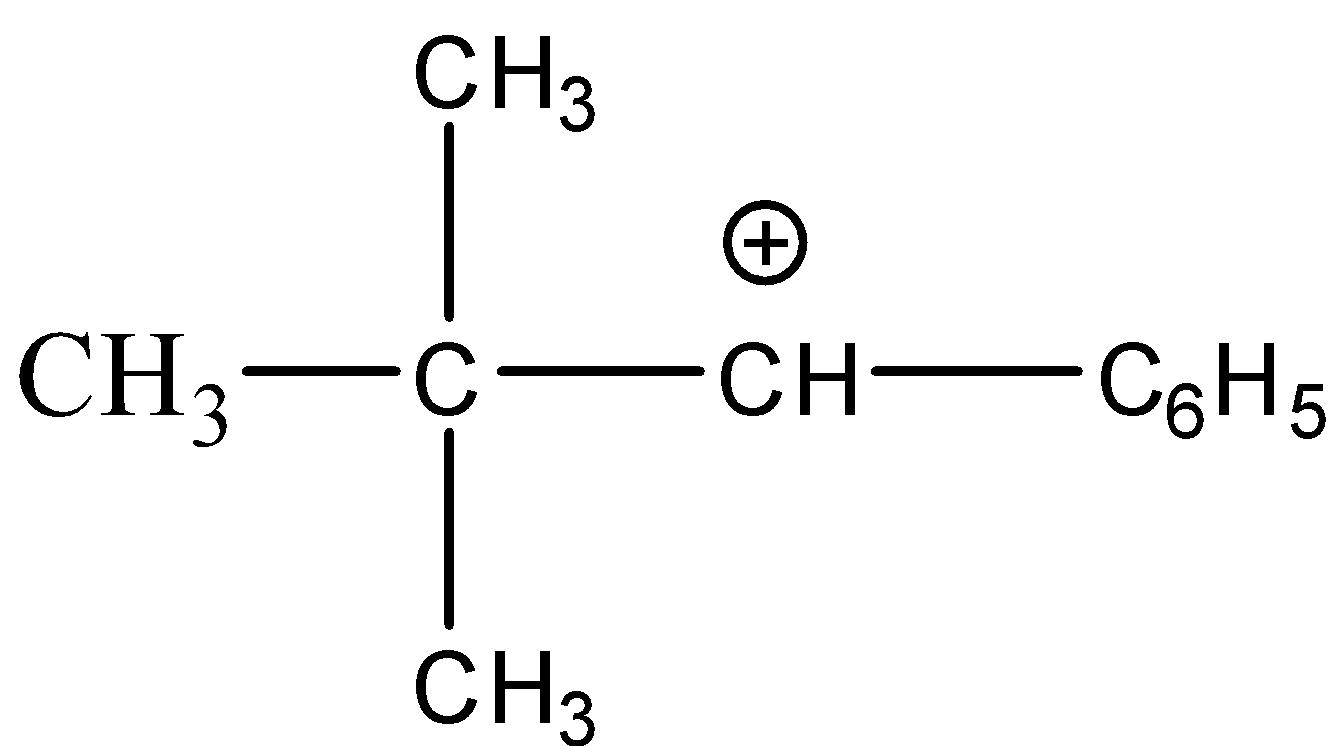
Solution
In order to find out which species is least stable we have to compare the stability order of these given carbocations. A carbocation is a species that contains a carbon atom with a positive charge and three bonds.
Complete step by step answer:
-Earlier, Carbocations were called carbonium ions. Now they are defined as any even-electron cation that possesses a particular positive charge on the carbon atom The carbon atom of the carbocation has sp2 hybridization and trigonal planar geometry.
-As carbocations are electron-deficient species due to incomplete octet, they are highly unstable and therefore reactive in nature. Hence, they are also called electrophiles.
-Based on the number of carbon groups that are bonded to the carbon, the carbocation can are divided into 4 types. These are methyl, primary, secondary or tertiary carbocation.
-In methyl carbocation, no carbon is bonded to the carbon-containing positive charge. In the case of primary, secondary, and tertiary carbocations the number of carbon groups attached to the carbon with the positive charge are 1,2 and 3 respectively. They are also known as 10,20 and 30 carbocation.
-The stability of these carbocations depends upon the hyper conjugative structures i.e. more the number of substituents bonded to the carbocation more will be the stability of the carbocation. Hence, the order of stability of carbocation is given as;
CH3+>10carbocation>20carbocation>30carbocation
-Hence, out of the given options, the carbocation that has the least number of substituents is CH3−CH2−CH2+. So, it will be the least stable species.
Therefore, Option (B) is correct.
Note:
As CH3 groups are electron-donating groups, substitution helps in decreasing the electron poverty of the carbocation atom. Hence Substituted carbocations are more stable than less substituted carbocations.
