Question
Question: Which of the following is known as invert soap? a.) Pentaerythritol monostearate b.) Trimethyl s...
Which of the following is known as invert soap?
a.) Pentaerythritol monostearate
b.) Trimethyl stearyl ammonium bromide
c.) Ethoxylated nonylphenol
d.) Sodium stearyl sulphate
Solution
Invert soap is a type of synthetic detergent in which the active part of the surface of the molecule is positively charged (cation). So, cationic detergent is called invert soap. Cation means positively charged ions.
Complete step by step solution:
Invert soap contains a surface which is positively charged, in normal soap a negatively charged surface is the active part.
Coming to the given options, option A, Pentaerythritol monostearate. The molecular formula of
Pentaerythritol monostearate is C23H46O5. It does not have an active part on the surface of the molecule. So, it is not the correct option.
Coming to option B, Trimethyl stearyl ammonium bromide.
The structure of the Pentaerythritol monostearate is as follows.
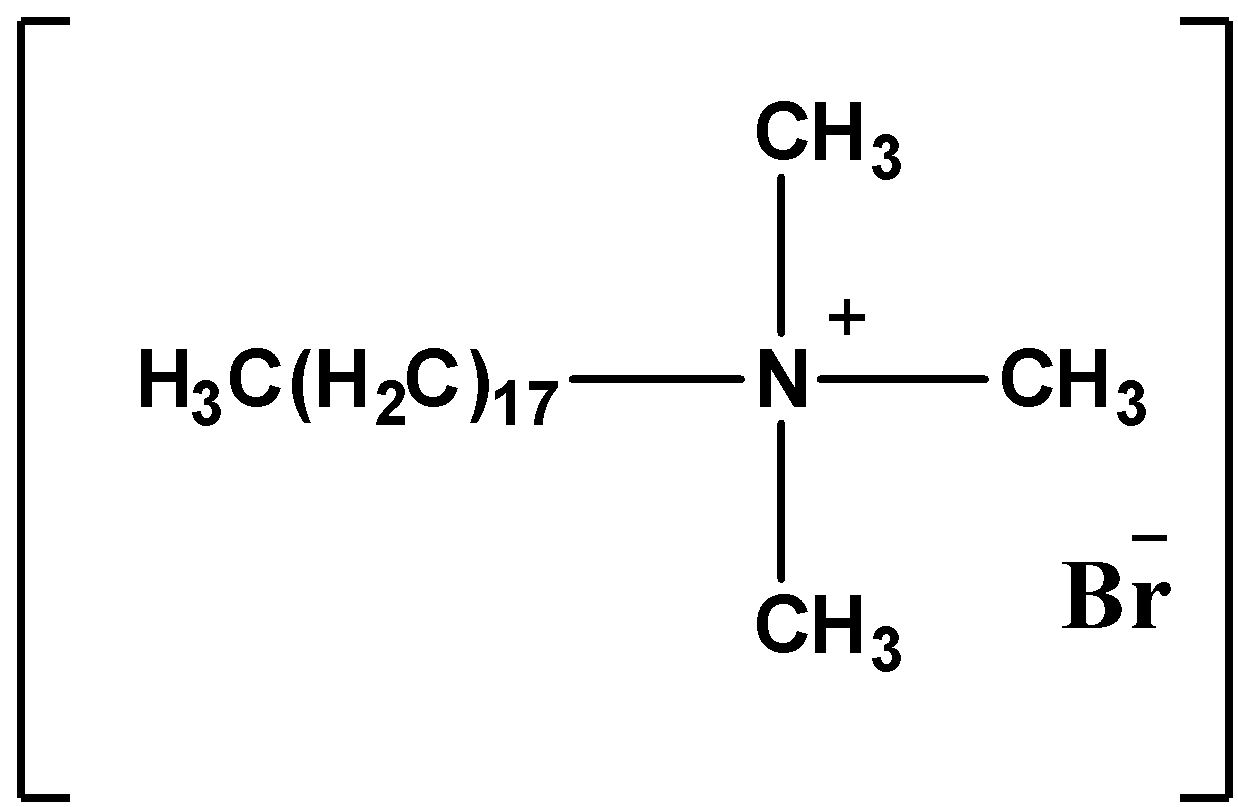
Trimethyl stearyl ammonium bromide contains quaternary ammonium salt with an active cation part on the surface of the molecule. So, Trimethyl stearyl ammonium bromide is invert soap.
Coming to option C, Ethoxylated nonylphenol, it is non-ionic in water and it is called non-ionic detergent. So, it is not correct.
Coming to option D, Sodium stearyl sulphate. The name itself says that it is anionic detergent because sodium is positively charged and stearyl sulphate is negatively charged means it has an anion active surface. So, it is not the correct option.
Therefore the correct answer for invert soap is Trimethyl stearyl ammonium bromide.
So, the correct option is B.
Note: Normal soap contains anion active surface but in case of invert soap, the cation part of the molecule is active. That is why normal soap is different from invert soap. Sodium stearyl sulphate is an example for normal soap.
