Question
Question: Which of the following is incorrectly match?...
Which of the following is incorrectly match?
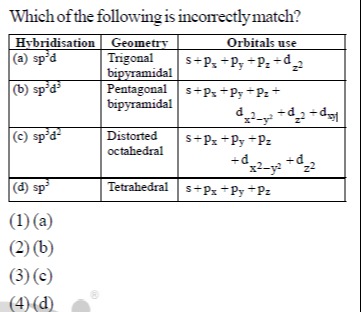
sp³d | Trigonal bipyramidal | s+pₓ+pᵧ+p₂ + d₂²
sp³d³ | Pentagonal bipyramidal | s+pₓ+pᵧ+p₂ + dₓ²₋ᵧ² + d₂² + dₓᵧ
sp³d² | Distorted octahedral | s+pₓ+pᵧ+p₂ + dₓ²₋ᵧ² + d₂²
sp³ | Tetrahedral | s+pₓ+pᵧ+p₂
(3)
Solution
The question asks to identify the incorrectly matched option. For sp³d² hybridization, the ideal electron geometry is Octahedral. The d-orbitals involved are dₓ²₋ᵧ² and d₂², which are correctly listed. However, the geometry is stated as "Distorted octahedral." While specific molecules with sp³d² hybridization can have distorted octahedral molecular geometries (e.g., square pyramidal, square planar due to lone pairs), the general geometry corresponding to sp³d² hybridization is Octahedral. Therefore, the match in option (c) is incorrect.
