Question
Question: Which of the following is incorrect regarding benzyl chloride? A.) It gives white precipitate with...
Which of the following is incorrect regarding benzyl chloride?
A.) It gives white precipitate with alcoholic AgNO3
B.) It is an aromatic compound with substitution in the side chain.
C.) It undergoes nucleophilic substitution reaction.
D.) It is less reactive than vinyl chloride.
Solution
In this question, benzyl chloride has the chemical formula as C6H5CH2Cl. In benzyl chloride, chlorine is not directly attached to the benzene ring. Also, in vinyl chloride, it undergoes resonance and gives two canonical structures.
Complete step by step answer:
As we know that the benzyl chloride compound can be written as C6H5CH2Cl. It is also known as chlorotoluene.
For option A.) , When benzyl chloride (C6H5CH2Cl) reacts with alcoholic silver nitrate (AgNO3) then in products silver chloride (AgCl) is formed in the product which is a white precipitate. This reaction can be written as:
C6H5CH2Cl+AgNO3C2H5OHC6H5CH2OC2H5+HNO3+AgCl
White ppt.
For option B.) , The benzyl chloride is an organic compound containing benzene ring and methyl chloride side chain as a substitution. The structure of benzyl chloride can be represented as:
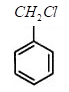
For option C.), The benzyl chloride undergoes nucleophilic substitution reaction. This is because chlorine is a very good leaving group and when it leaves then a carbocation is formed on which a nucleophile can easily attack.
For option D.), Benzyl chloride is more reactive than the vinyl chloride. Here, vinyl chloride can be written as CH2=CHCl. The chlorine is not reactive in vinyl chloride because of resonance stabilization. The lone pair of chlorine participates in resonance to give two resonating structures. These structures can be represented as:
[CH2=CH−Cl..↔C−H2CH=Cl+]
As we can see , vinyl chloride chlorine gives its lone pair and forms two resonating structures.
So the correct answer is option D
Note: Always remember that in benzyl chloride, chlorine leaves the benzyl chloride to form stable cation hence the compound reacts easily and in vinyl chloride chlorine donates lone pair due to which compound becomes less stable which decreases its reactivity.
