Question
Question: Which of the following is incorrect about \({\text{NaCl}}\) structure? A) Nearest neighbours of \(...
Which of the following is incorrect about NaCl structure?
A) Nearest neighbours of Na+ ion is six Cl− ion.
B) Na+ ions make FCC lattice.
C) Cl− ions are packed in a cubic close packed arrangement.
D) There are eight next nearest neighbours of Na+ ion.
Solution
We know that the smallest repeating unit of a crystal lattice is known as a unit cell. In a face centered unit cell, atoms are present at the centre of all the six faces of the cube and at all the eight corners of the cube.
Complete step by step answer:
We know that NaCl has a cubic unit cell. Thus, the Na+ and Cl− ions are closely packed.
The formula of NaCl shows that the Na+ and Cl− ions are present in the 1:1 ratio.
Na+ ions occupy octahedral voids in the crystal lattice of NaCl. This is because the size of Na+ ions is smaller than that of Cl− ions. The Cl− ions are located at the corners and the centre of each face of the unit cell.
We know that the lattice of NaCl is face centered cubic (FCC). The coordination number of FCC is twelve and it contains four atoms per unit cell.
Each Na+ ion is surrounded by six Cl− ions and each Cl− ion is surrounded by six Na+ ions. Thus, the coordination number of each ion is six.
The structure of unit cell of NaCl is as follows:
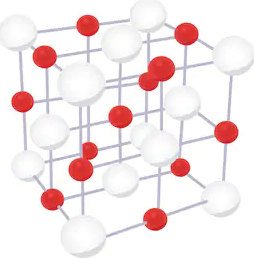
The white sphere represent Cl− ions and the red spheres represent Na+ ions.
Thus, the statement ‘there are eight next nearest neighbours of Na+ ion’ is incorrect.
So,Option D is correct.
Note: The sodium chloride structure is a face centered cubic structure. There are four atoms per unit cell of a face centered cubic lattice. Six chloride ions surround one sodium ion and six sodium ions surround one chloride ion. The packing efficiency of the FCC lattice is 74%.
