Question
Question: Which of the following is incorrect about \({{H}_{4}}{{P}_{2}}{{O}_{5}}\)? A. It contains P in +5 ...
Which of the following is incorrect about H4P2O5?
A. It contains P in +5 oxidation state
B. It is a dibasic acid
C. It is strongly reducing in nature
D. It contains one P−O−P bond
Solution
H4P2O5 is known by the name pyro phosphorus acid which is generally colorless or odorless in nature. H4P2O5 is soluble in water, diethyl ether, and ethyl alcohol. This acid is somewhat corrosive but not kept in the category of toxic acids.
Complete step by step answer:
We have to choose the incorrect statement about H4P2O5 this can be explained on the basis of discussion of each point.
A. It contains P in +5 oxidation state: Oxidation state of P i.e. Phosphorus is calculated as this way in H4P2O5; Hydrogen have -1 oxidation state, oxygen carries -2 charge and overall charge on the compound is zero so (−1)×4+x×2+(−2)×5=0 where we find the value of x = +3 so this statement is not true.
B. It is dibasic acid: H4P2O5 is dibasic acid this can be explained by the structure of H4P2O5 which is as follows:
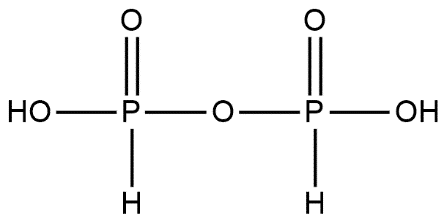
Hydrogen atoms which are attached with the oxygen atom contribute to the basicity here we can see that 2 hydrogens atoms are attached so it is dibasic in nature.
C. It is strongly reducing in nature: Given statement is true because it can easily donate electrons and become oxidized in nature.
D. It contains one P−O−P bond: The given statement is true; we can easily see in its structure that it has one P−O−P bond.
So, the correct answer is “Option A”.
Note: The anhydrous form of pyrophosphoric acid crystallizes in two polymorphs and this compound has no particular applications in main industries and pyrophosphoric acid is also known by the other name called diphosphoric acid.
