Question
Question: Which of the following is incorrect about a heterogeneous mixture? A. Constituents of heterogeneou...
Which of the following is incorrect about a heterogeneous mixture?
A. Constituents of heterogeneous mixtures are uniformly mixed.
B. It is composed of multiple separate parts.
C. It is composed of a chemical solution.
D. All of these
Solution
The physical combination of two or more substances without losing the identity of each substance is known as a mixture. A mixture can be a form of solution, suspensions and colloids. The substances in a mixture do not undergo a chemical change and it is not necessary to mix each element in a specific ratio to form a mixture.
Complete answer:
There are two primary types of mixture which are discussed below:
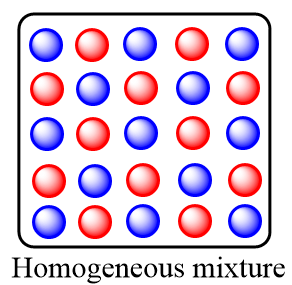
Homogeneous mixture: These are the type of mixtures in which the substances or components are mixed uniformly and distributed in a uniform composition throughout the mixture. Only one phase of matter is observed in the homogeneous mixtures. The homogeneous mixtures are also known as solutions.
Heterogeneous mixture: These are the type of mixtures in which the substances or components are completely mixed and are distributed non uniformly within the mixture. It consists of a non-uniform composition and there exists two or more phases of solution within the heterogeneous mixture.
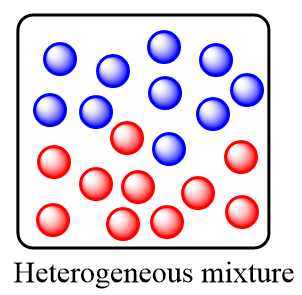
The key-points for a heterogeneous mixture are as follows:
1.Particles or components are distributed non uniformly.
2.It is composed of multiple separate parts i.e.; it consists of two or more phases of matter within the mixture.
3.Components of the mixture can be separated out physically i.e., it is not composed of a chemical solution.
Therefore, options (A) and (C) are the correct answers.
Note:
Remember that in homogeneous mixture, as the distribution of the components is uniform, so we cannot judge a homogeneous mixture by just looking at it whereas in a heterogeneous mixture, due to the non-uniform composition of substance, one can easily identify it by just looking at the mixture.
