Question
Question: Which of the following is false about \( {H_2}{O_2}. \) (A) Act as both oxidizing and reducing ag...
Which of the following is false about H2O2.
(A) Act as both oxidizing and reducing agent
(B) Two OH bond lie in same plane
(C) Pale blue liquid
(D) Can be oxidized by ozone
Solution
H2O2 also known as hydrogen peroxide has number of uses in chemistry like bleaching agent, antiseptic agent etc. and it contains a peroxide bond (which is Oxygen-Oxygen bond) and it does not have a planar structure because the molecule stabilizes in such a way that structure becomes non-planar.
Complete step by step solution:
H2O2 known as hydrogen peroxide is a colorless pale blue liquid in which peroxide bond is available (single oxygen-oxygen) it is formed when two molecules of hydrogen combine with two molecules of oxygen.
Some of the basic uses of H2O2 are like using bleaching agents, oxidizer, and antiseptic.
If we discuss some of its chemical properties then, those are as follows: -
It acts as both oxidizing agent as well as reducing agent which can be clear by these following reactions: - Oxidation Reaction: - Na2SO3+ H2O2→ Na2SO4+ H2O
Reduction Reaction: - BaO2+ H2O2→ BaO + H2O + O2
It can be easily oxidized by ozone as well. This is cleared by looking at the reaction of Hydrogen peroxide with ozone.
O3+ H2O2→ H2O + 2O2
Hence from this we can say ozone oxidizes hydrogen peroxide as well.
Hydrogen peroxide gets decomposed when exposed to sunlight, hence it is kept in dark bottles.
Structure of Hydrogen peroxide: -
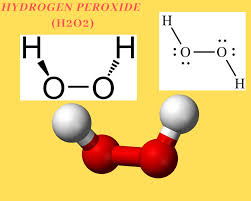
OH bond back of the plane
OH bond out of the plane
Hence by looking at the structure of Hydrogen peroxide, we can say the 2OH groups present are in different planes.
Therefore options A, C, D are correct.
Note:
In this question, specific properties of Hydrogen peroxide were asked and there are other few important chemicals like H2S gas, Halogen compounds, Peracids and super-acids which are also important and their few basic properties are good to know which helps in answering similar such questions.
