Question
Question: Which of the following is example of cyclic silicate?...
Which of the following is example of cyclic silicate?
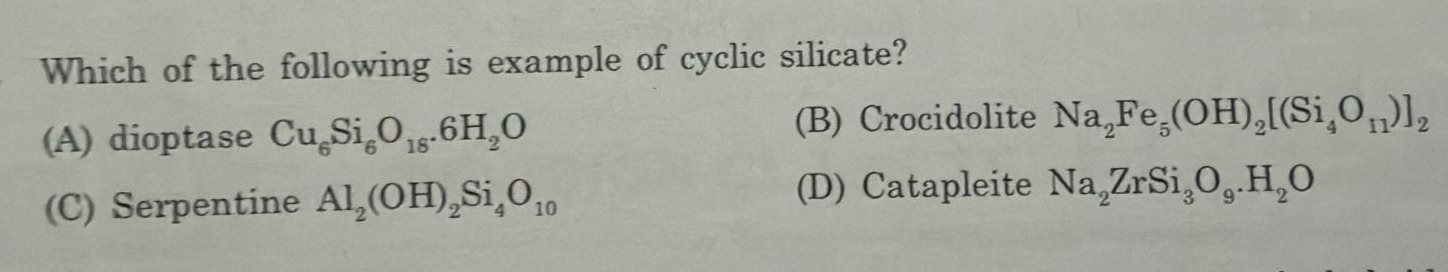
dioptase Cu6Si6O18.6H2O
Crocidolite Na2Fe5(OH)2[(Si4O11)]2
Serpentine Al2(OH)2Si4O10
Catapleite Na2ZrSi3O9.H2O
dioptase Cu6Si6O18.6H2O
Solution
Cyclic silicates, also known as ring silicates, are characterized by the cyclic arrangement of SiO4 tetrahedra. These rings are formed by sharing two oxygen atoms between adjacent tetrahedra. The general formula for the silicate anion in cyclic silicates is (SiO3)n2n−, where n is the number of tetrahedra in the ring, typically n=3, 4, or 6. This formula implies a Si:O ratio of 1:3 in the silicate anion.
-
Dioptase Cu6Si6O18.6H2O: The silicate anion is Si6O18. The ratio of Si:O is 6:18 = 1:3. This fits the general formula (SiO3)612−, corresponding to a six-membered ring structure. Thus, Dioptase is a cyclic silicate.
-
Crocidolite Na2Fe5(OH)2[(Si4O11)]2: The silicate anion is (Si4O11)2=Si8O22. The ratio of Si:O is 8:22 = 4:11. This corresponds to double chain silicates (amphiboles), not cyclic silicates.
-
Serpentine Al2(OH)2Si4O10: The silicate anion is Si4O10. The ratio of Si:O is 4:10 = 2:5. This corresponds to sheet silicates (phyllosilicates), not cyclic silicates.
-
Catapleite Na2ZrSi3O9.H2O: The silicate anion is Si3O9. The ratio of Si:O is 3:9 = 1:3. This fits the general formula (SiO3)36−, corresponding to a three-membered ring structure. Thus, Catapleite is also a cyclic silicate.
Based on the analysis, both Dioptase and Catapleite are examples of cyclic silicates. However, Dioptase, containing the Si6O18 ring, is a well-known example.
