Question
Question: Which of the following is electron deficient? A.\({(C{H_3})_2}\) B.\({(Si{H_3})_2}\) C.\({(B{H...
Which of the following is electron deficient?
A.(CH3)2
B.(SiH3)2
C.(BH3)2
D.PH3
Solution
The valence electrons present in carbon is four, the valence electrons present in silicon is four, the valence electron in boron is three and the valence electron in phosphorus is five.
Complete step by step answer:
The structure of (CH3)2 is shown below.
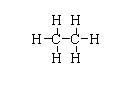
The atomic number of carbon is 6. The electronic configuration is [He]2s22p2. Hence, only four electrons take part in bond formation.
In (CH3)2, all the valency of carbon is fulfilled so there is no electron deficiency.
The structure of (SiH3)2 is shown below.
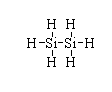
The atomic number of silicon is 14. The electronic configuration is [Ne]3s23p2. Hence, the valence electron in silicon is four.
In (SiH3)2, all the valency of silicon is fulfilled so there is no electron deficiency.
The structure of (BH3)2 is shown below.
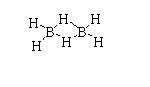
The atomic number of boron is 5. The electronic configuration is [He]2s22p1. The valence electron in boron is 3.
In diborane, fourteen electrons are needed to form seven bonds but only twelve bonds are present. Due to this it forms a special type of structure.
Thus, diborane is electron deficient.
The structure of PH3 is shown below.
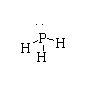
The atomic number of phosphorus is 17. The electronic configuration is [Ne]3s23p3. There are five valence electrons in phosphorus.
In PH3, three electrons take part in bonding and two nonbonding lone pairs are present.
Hence, in PH3 all the valency is fulfilled, therefore it is not electron deficient.
Thus, the correct answer is option C.
Note:
When the compound boron hydride BH3 undergoes dimerizes to form diboraneB2H6 , each boron atom contains only six electrons, thus its octet is incomplete.
