Question
Question: Which of the following is aromatic in nature? 
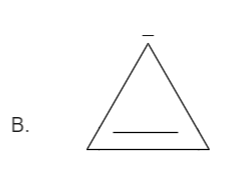
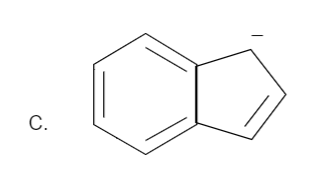
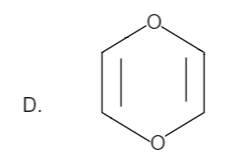
Solution
The aromatic compounds are those which have one more ring in its structure and it contains pi electrons which are delocalized all over the structure. Also, the aromaticity of a compound can be found with the help of Huckel’s rule. According to which a compound which contains (4n+2) pi electrons is considered in nature. Thus we will analyze each compound.
Formula Used:
For aromatic compounds, the number of pi electrons must be (4n+2), where n is an integer starting from zero.
Complete Answer:
Aromatic compounds are those compounds that contain one more ring and follow the Huckel rule of aromaticity. According to the Huckel rule of aromaticity, a planar ring molecule will be aromatic in nature when it contains a number of pi electrons in order of (4n+2). We will analyze each compound respectively. Each double bond constitutes two electrons and a negative charge also indicates two electrons.
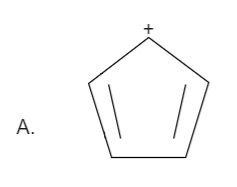
Here the total number of electrons are (2+2=4). Thus it is not in order of (4n+2), therefore it is not an aromatic compound.
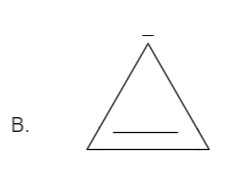
Total number pi bond is one and it has a negative charge also which in total has (2+2=4) electrons. Thus it is not in order of (4n+2), therefore it is not an aromatic compound.
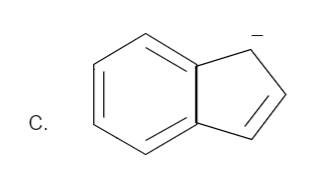
Total number of pi bonds is four and it has a negative charge also. Therefore the total number of electrons is: n=2 which is in the order of (4n+2) for n=2. Hence it is an aromatic compound.
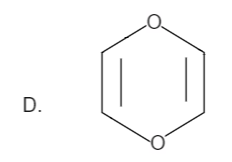
Here the total number of electrons are (2+2=4). Thus it is not in order of (4n+2), therefore it is not an aromatic compound.
Hence the correct option is C.
Note:
It must be noted that each pi bond constitutes two electrons and a negative charge accounts for two electrons where a positive charge does not account for any electron. This is because a negative charge is developed when atoms accept electrons and a positive charge is developed when atoms lose electrons. Also for aromatic compounds, the compound must be planar.
