Question
Question: Which of the following is aromatic in nature? A.
B. 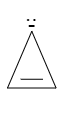
C. 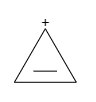
D.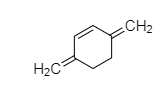
E. None of these
Solution
Hint : Compounds that contain one or more isolated or fused benzene rings are called aromatic compounds.
When the bonding orbital/electron is not restricted to only two atoms but is spread over more than two atoms then, such orbitals/electrons are called delocalized and this phenomenon is known as delocalization.
Complete step by step solution :
Hückel's rule estimates whether a compound will have aromatic properties or not. Criteria for simple aromatics are:
The molecule must have 4(n+2)Πe− in the delocalized p-orbital cloud.
The molecule must be able to be planar and are cyclic.
Each atom making up the cyclic compound must be able to participate in delocalizing the electrons by having a p-orbital or an unshared pair of electrons.
It is a cyclic compound and it has conjugate but Huckel’s rule 4(n+2)Πe− rule is not followed. Also, this structure is not a plan. Hence, it is non-aromatic in nature.
As there is an extra lone pair of electrons present so the total number of electrons comes out to be 4Πe−. It is also cyclic. Hence, it is anti-aromatic in nature.
It is cyclic and planer. Also, due to the presence of 4(n+2)Πe−, it follows Huckel’s rule and therefore, it is aromatic in nature.
It is cyclic but it is not planer and it has 4Πe− in conjugation but not in the ring. Hence, it is non-aromatic in nature.
So, the correct answer is “Option C”.
Note : The possibility to make a mistake is that you may choose option B. As it is cyclic and planar but it does not follow Huckel’s rule, so it is antiaromatic, not aromatic in nature.
