Question
Question: Which of the following is aromatic 1.
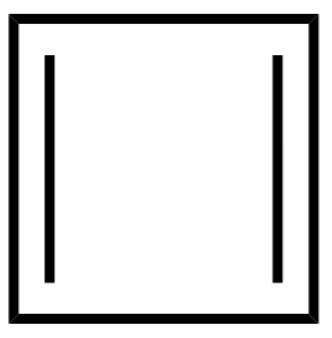
2.
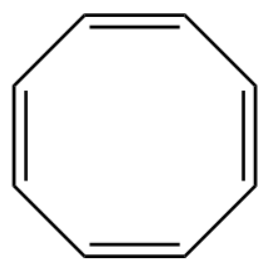
3.
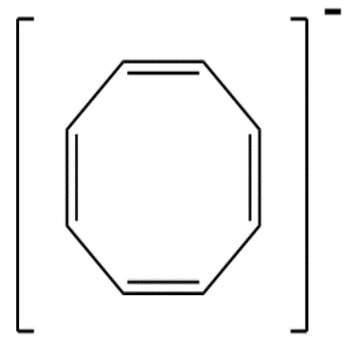
4. 
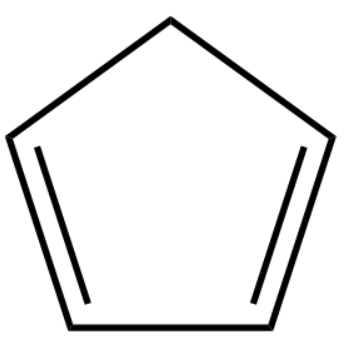
5.
Solution
When we have to find the aromaticity of a compound we use the Huckel’s rule of (4n+2π) electrons where n can be any integer with a positive value, including zero. So the number of pi-electrons in an aromatic molecule must be equal to 2,6,10,14,18 …. and so on.
Complete answer:
Aromaticity is the property of a cyclic, planar molecule with a ring resonance bonds that exhibits more stability than other arrangements of atoms.
For a molecule to exhibit aromaticity there are following condition it must met:
i.It should be cyclic and every atom in the cyclic ring must be conjugated which will provide a delocalised pi-electron system. This further translates that the cyclic ring must have an empty p orbital and must be capable of participating in resonance.
ii.They must follow Huckel’s rule.
iii.The molecule should be planar.
Let’s understand Huckel's rule. We all have studied about benzene. In fact whenever we talk about aromatic compounds benzene is the first name that pops up in our mind. Using Huckel’s rule lets understand why benzene is an aromatic compound. Since the number of pi-electrons in benzene is 6 , so the value of n corresponds to 1 , now we will simply put this value in the Huckel’s rule formula that is (4n+2π) .
We get
[(4×1)+2]=6
Thus benzene obeys Huckel’s rule.
Let’s look into the options provided to us:
1.Cyclooctatetraene
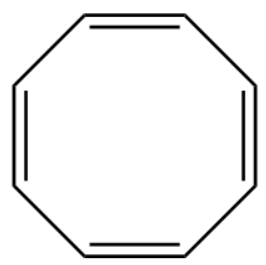
In this molecule of cyclooctatetraene it fails to satisfy the (4n+2π) electron rule as it has 8−pi -electrons so the value of n corresponds to 23 which is not an integer. It is best described as non-aromatic.
2.Cyclobutadiene
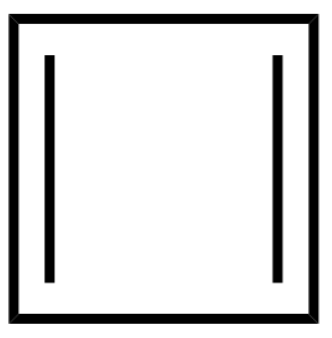
Cyclobutadiene also doesn’t satisfy the (4n+2π) where the number of pi-electrons is 4 , therefore the value of n is not an integer. It is an antiaromatic compound.
3.Cyclopentadiene
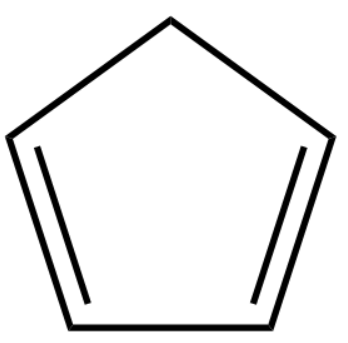
4.Cyclopentadiene possess 4−pi -electron system therefore its non-aromatic as it doesn’t satisfy the Huckel’s rule.
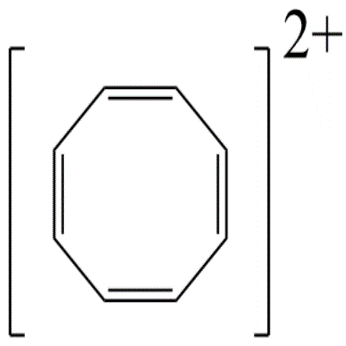
5.Cyclooctatetraene anion contains 10−pi -electrons since a ring contains maximum of only 6−pi -electrons therefore it is not aromatic.
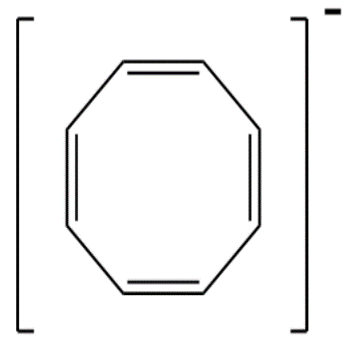
Therefore the answer is 1 .
Note:
A negative charge or anion corresponds to 2−pi -electron. While checking the aromaticity look for the above mentioned criteria. If any molecule doesn’t obey any of these, it is not aromatic in nature.
