Question
Question: Which of the following is/are polar in nature? A.
B.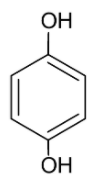
C.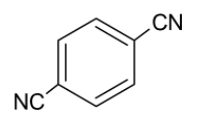
D.Both B and C
Solution
Chemical compounds in which the constituent atoms form a polar covalent bond are said to be polar in nature. A bond is polar when there is a difference in the electronegativities of the atoms and the bond has unequal sharing of electrons as a result.
Complete step-by-step answer: The more electronegative atom pulls electrons towards it and the dipole moment of the bond is said to be towards that molecule.
Considering option A, we have para dichloro benzene.
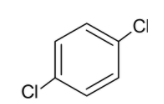
We know that chlorine is more electronegative than carbon and thus the dipole moment of the bond is directed towards chlorine atom. But the dipole moments of both the carbon chlorine bonds are opposite to each other and cancel out making the dipole moment of the compound zero.
Considering option B, we have hydroquinone
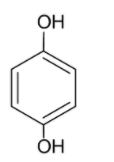
The dipole moments of the carbon oxygen bond and the oxygen hydrogen bonds are directed towards oxygen. The carbon oxygen dipole moments are opposite to each other but the arrangement of the hydrogen atom is not symmetrical. Thus, the molecule is polar.
Considering option C,
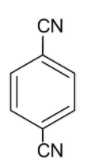
The dipole moments of the carbon nitrogen bond and the carbon (ring) - carbon (cyanide) bonds are directed towards nitrogen and cyanide groups respectively. The carbon-carbon dipole moments are opposite to each other but the arrangement of the nitrogen atom is not symmetrical. Thus, the molecule is polar.
Hence, the correct answer is D.
Note: Polarity or the polar nature of a bond or a molecule is measured in terms of dipole moment. We know that the overall polarity of a molecule depends upon the structure of the molecule and placement of the polar bonds. The combined resultant of the dipoles of all bonds determines the overall polarity of the molecule.
