Question
Question: Which of the following is/are formed by the reaction of benzaldehyde with aqueous NaOH followed by a...
Which of the following is/are formed by the reaction of benzaldehyde with aqueous NaOH followed by acidification with dilute hydrochloric acid?
A. Benzoic acid
B. Benzyl alcohol
C. Benzoic acid and benzyl alcohol
D. p-hydroxybenzaldehyde
Solution
The aqueous solution of sodium hydroxide causes disproportionation of two aldehyde molecules. The aldehyde molecules that do not have alpha hydrogen like benzaldehyde give the disproportionation reaction. By the disproportionation one aldehyde molecule reduces to alcohol. Second aldehyde molecule oxidized to acid.
Complete answer:
Two molecules of benzaldehyde the alkali solution gives the disproportionation reaction which is known as Cannizzaro reaction.
In the Cannizzaro reaction, two aldehyde molecules react in presence of a base and form a carboxylic acid and alcohol.
The base attacks the carbonyl group of one benzaldehyde molecule and forms a tetrahedral anion.
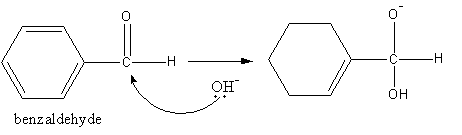
The tetrahedral anion forms acid and removes a hydride. This hydride attacks the carbonyl group of another benzaldehyde molecule.
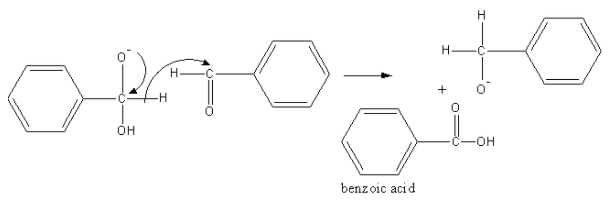
Another anion abstract proton from acid and form alcohol and acid anion get acidified with dilute hydrochloric acid, so an acid and alcohol form.
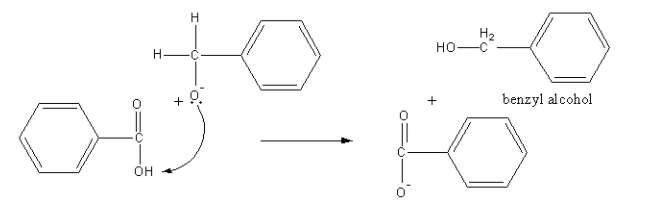
So, benzoic acid and benzyl alcohol are formed by the reaction of benzaldehyde with aqueous NaOH followed by acidification with dilute hydrochloric acid.
**Therefore, option (C) Benzoic acid and benzyl alcohol, is correct.
Note:**
In the Cannizzaro reaction, nucleophilic attacks take place on aldehyde, so the aldehyde having no alpha-hydrogen gives Cannizzaro reaction. If the aldehyde has alpha-hydrogen, the aldehyde undergoes keto-enol tautomerism which decreases the electrophilicity of the carbonyl group, so the nucleophilic attacks decrease. The alpha-hydrogen having aldehydes gives an aldol condensation reaction. The products of aldol condensation reaction are beta-hydroxy ketone and beta-hydroxy aldehyde.
