Question
Question: Which of the following is/are correctly matched? a.) \[[Ni{{(CO)}_{4}}]-ds{{p}^{2}}\] and diamagne...
Which of the following is/are correctly matched?
a.) [Ni(CO)4]−dsp2 and diamagnetic
b.) [Ni(en)3](NO2)2−sp3d2 and two unpaired electrons
c.) [V(NH3)6]Cl3−sp3d2 and two unpaired electrons
d.) [Mn(NO+)3(CO)]−sp3and diamagnetic
Solution
In order to solve this question, check the oxidation state of the central atom of each compound. Using that, predict the compound’s hybridization. If any orbital contains unpaired electrons, it is paramagnetic, otherwise it is diamagnetic.
Complete step by step solution:
Let us look at all the compounds one by one.
[Ni(CO)4]
Oxidation state of Ni = 0.
Its electronic configuration is - [Ar]3d84s2
Carbon monoxide is a strong field ligand. Therefore, pairing of electrons takes place. This can be represented as –
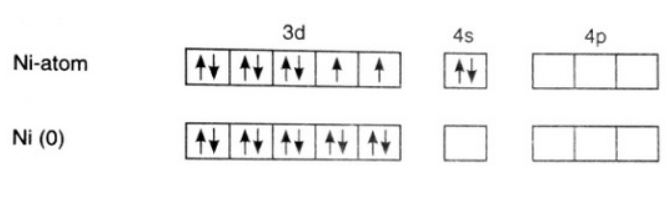
The hybridization of the compound is – sp3.
As all electrons in this compound are paired, it is a diamagnetic compound.
Option (a) is incorrect.
[Ni(en)3](NO2)2
Oxidation state of Ni = +2
Its electronic configuration is - [Ar]3d84s0
Ethylene diamine is a weak field ligand. Hence, there is no pairing of electrons. This can be represented as –
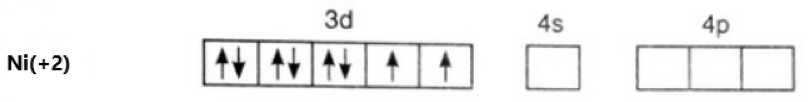
The hybridization of the compound is – sp3d2.
As we can see, 2 electrons in 3d are unpaired, it is a paramagnetic compound.
Option (b) is correct.
[V(NH3)6]Cl3
Oxidation state of V = +3
Its electronic configuration is - [Ar]3d24s0
Ammonia is a strong field ligand. Therefore, pairing of electrons takes place. This can be represented as –
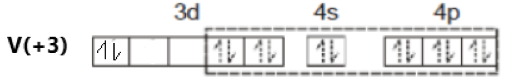
The hybridization of the compound is – d2sp3.
As all electrons in this compound are paired, it is a diamagnetic compound.
Option (c) is incorrect.
[Mn(NO+)3(CO)]
Oxidation state of Mn = +2
Its electronic configuration is - [Ar]3d54s0
Nitrosonium is a weak field ligand. Hence, there is no pairing of electrons. This can be represented as –
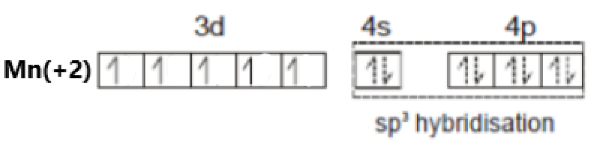
The hybridization of the compound is – sp3.
As all electrons in this compound are unpaired, it is a paramagnetic compound.
Option (d) is incorrect.
Therefore, the answer is – option (b).
Note: Compounds not attracted to magnetic fields are known as diamagnetic compounds. They have all paired electrons. Whereas, compounds which are attracted to magnetic fields, are known as paramagnetic compounds. They have unpaired electrons.
