Question
Question: Which of the following is /are an example of orthosilicate? (This question has multiple correct answ...
Which of the following is /are an example of orthosilicate? (This question has multiple correct answers)
(a)- ZrSiO4
(b)- Mg2SiO4
(c)- Zn3Si2O7
(d)- Asbestos
Solution
Orthosilicates are the group of compounds in the chemistry in which silicate is the anion part in which silicon is attached with four oxygen atoms through a single bond and has a 4- charge. So, check all the compounds, if the compound has [SiO4]4− anion, then it is an orthosilicate.
Complete step-by-step answer:
Orthosilicates are also known as silicon tetroxide anion. The structure of the silicate ion in the orthosilicate is given below:
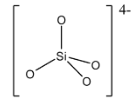
Since, the silicate ion [SiO4]4− is the anion part, the cations like sodium, magnesium, etc can form salts with this and are called as orthosilicate.
Let us check all the options.
(a)- ZrSiO4: This compound is known as Zirconium silicate, it is also known as Zirconium orthosilicate because the ions in this are in the form Zr4+ and [SiO4]4−. The structure is given below:
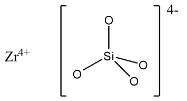
(b)- Mg2SiO4: This compound is magnesium orthosilicate in which magnesium is the cation and [SiO4]4− is the anion. The structure is given below:
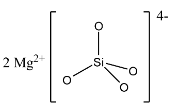
(c)- Zn3Si2O7: This compound is zinc pyrosilicate. The pyrosilicate doesn’t have [SiO4]4− anion.
(d)- Asbestos: It is also a silicate of magnesium but it is not an orthosilicate because the formula of asbestos is Mg3Si2O5(OH)4 .
Therefore, the correct answers are an option (a) and option (b).
Note: It must be noted that not all the silicates are orthosilicate, only those compounds in which the anion is [SiO4]4− will be orthosilicate. Since asbestos is used for many purposes but it is a cancer-causing substance.
